Internalization of IgG-coated targets results in activation and secretion of soluble CD40 ligand and RANTES by human platelets
- PMID: 21177916
- PMCID: PMC3067351
- DOI: 10.1128/CVI.00296-10
Internalization of IgG-coated targets results in activation and secretion of soluble CD40 ligand and RANTES by human platelets
Abstract
Platelets are crucial elements for maintenance of hemostasis. Other functions attributable to platelets are now being appreciated, such as their role in inflammatory reactions and host defense. Platelets have been reported to bind immunological stimuli like IgG complexes, and for nearly 50 years it has been speculated that platelets may participate in immunological reactions. Platelets have been reported to bind and internalize various substances, similar to other leukocytes, such as macrophages and dendritic cells. In the present study, we tested the hypothesis that human platelets can bind and internalize IgG-coated particles, similar to leukocytes. To this end, we observed that interaction with IgG-coated beads resulted in platelet activation (as measured by CD62P expression), internalization of targets, and significant soluble CD40 ligand (sCD40L) and RANTES (regulated upon activation, normal T cell expresses and secreted) secretion. Blocking FcγRIIA with monoclonal antibody (MAb) IV.3 or inhibiting actin remodeling with cytochalasin D inhibited platelet activation, internalization, and cytokine production. These data suggest that platelets are capable of mediating internalization of IgG-coated particles, resulting in platelet activation and release of both sCD40L and RANTES.
Figures

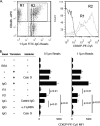
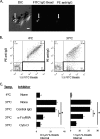

References
-
- Antczak, A. J., N. Singh, S. R. Gay, and R. G. Worth. 2010. IgG-complex stimulated platelets: a source of sCD40L and RANTES in initiation of inflammatory cascade. Cell. Immunol. 263:129-133. - PubMed
-
- Boehlen, F., and K. J. Clemetson. 2001. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus. Med. 11:403-417. - PubMed
-
- Brandt, E., A. Ludwig, F. Petersen, and H. D. Flad. 2000. Platelet-derived CXC chemokines: old players in new games. Immunol. Rev. 177:204-216. - PubMed
-
- Bubel, S., D. Wilhelm, M. Entelmann, H. Kirchner, and H. Kluter. 1996. Chemokines in stored platelet concentrates. Transfusion 36:445-449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

