Somatosensory context alters auditory responses in the cochlear nucleus
- PMID: 21178001
- PMCID: PMC3295206
- DOI: 10.1152/jn.00807.2010
Somatosensory context alters auditory responses in the cochlear nucleus
Abstract
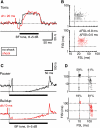
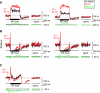
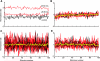
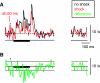
The cochlear nucleus, the first central auditory structure, performs initial stimulus processing and segregation of information into parallel ascending pathways. It also receives nonauditory inputs. Here we show in vivo that responses of dorsal cochlear nucleus (DCN) principal neurons to sounds can change significantly depending on the presence or absence of inputs from the somatosensory dorsal column nucleus occurring before the onset of auditory stimuli. The effects range from short-term suppression of spikes lasting a few milliseconds at the onset of the stimulus to long-term increases or decreases in spike rate that last throughout the duration of an acoustic stimulus (up to several hundred milliseconds). The long-term effect requires only a single electrical stimulus pulse to initiate and seems to be similar to persistent activity reported in other parts of the brain. Among the DCN inhibitory interneurons, only the cartwheel cells show a long-term rate decrease that could account for the rate increases (but not the decreases) of DCN principal cells. Thus even at the earliest stages of auditory processing, the represented information is dependent on nonauditory context, in this case somatosensory events.
Figures

References
-
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in the mormyrid electrosensory lobe. J Exp Biol 202: 1339–1347, 1999 - PubMed
-
- Berrebi AS, Mugnaini E. Distribution and targets of the cartwheel cell axon in the dorsal cochlear nucleus of the guinea pig. Anat Embryol 183: 427–454, 1991 - PubMed
-
- Davis KA, Miller RL, Young ED. Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus. J Neurophysiol 76: 3012–3024, 1996 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

