Transplantation tolerance to a single noninherited MHC class I maternal alloantigen studied in a TCR-transgenic mouse model
- PMID: 21178009
- PMCID: PMC3774109
- DOI: 10.4049/jimmunol.1003023
Transplantation tolerance to a single noninherited MHC class I maternal alloantigen studied in a TCR-transgenic mouse model
Abstract
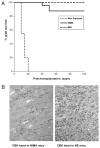
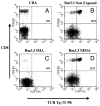
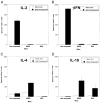
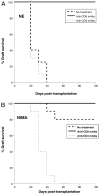
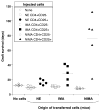
The mechanisms underlying tolerance to noninherited maternal Ags (NIMA) are not fully understood. In this study, we designed a double-transgenic model in which all the offspring's CD8(+) T cells corresponded to a single clone recognizing the K(b) MHC class I protein. In contrast, the mother and the father of the offspring differed by the expression of a single Ag, K(b), that served as NIMA. We investigated the influence of NIMA exposure on the offspring thymic T cell selection during ontogeny and on its peripheral T cell response during adulthood. We observed that anti-K(b) thymocytes were exposed to NIMA and became activated during fetal life but were not deleted. Strikingly, adult mice exposed to NIMA accepted permanently K(b+) heart allografts despite the presence of normal levels of anti-K(b) TCR transgenic T cells. Transplant tolerance was associated with a lack of a proinflammatory alloreactive T cell response and an activation/expansion of T cells producing IL-4 and IL-10. In addition, we observed that tolerance to NIMA K(b) was abrogated via depletion of CD4(+) but not CD8(+) T cells and could be transferred to naive nonexposed mice via adoptive transfer of CD4(+)CD25(high) T cell expressing Foxp3 isolated from NIMA mice.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

References
-
- Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. - PubMed
-
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. - PubMed
-
- Vernochet C, Caucheteux SM, Kanellopoulos-Langevin C. Bidirectional cell trafficking between mother and fetus in mouse placenta. Placenta. 2007;28:639–649. - PubMed
-
- van Rood JJ, Claas F. Both self and non-inherited maternal HLA antigens influence the immune response. Immunol Today. 2000;21:269–273. - PubMed
-
- Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

