Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial
- PMID: 21178763
- PMCID: PMC3232054
- DOI: 10.1097/SLA.0b013e3181fcdb22
Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial
Abstract
Objective: To determine whether out-of-hospital administration of hypertonic fluids would improve survival after severe injury with hemorrhagic shock.
Background: Hypertonic fluids have potential benefit in the resuscitation of severely injured patients because of rapid restoration of tissue perfusion, with a smaller volume, and modulation of the inflammatory response, to reduce subsequent organ injury.
Methods: Multicenter, randomized, blinded clinical trial, May 2006 to August 2008, 114 emergency medical services agencies in North America within the Resuscitation Outcomes Consortium.
Inclusion criteria: injured patients, age ≥ 15 years with hypovolemic shock (systolic blood pressure ≤ 70 mm Hg or systolic blood pressure 71-90 mm Hg with heart rate ≥ 108 beats per minute). Initial resuscitation fluid, 250 mL of either 7.5% saline per 6% dextran 70 (hypertonic saline/dextran, HSD), 7.5% saline (hypertonic saline, HS), or 0.9% saline (normal saline, NS) administered by out-of-hospital providers. Primary outcome was 28-day survival. On the recommendation of the data and safety monitoring board, the study was stopped early (23% of proposed sample size) for futility and potential safety concern.
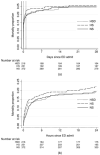
Results: : A total of 853 treated patients were enrolled, among whom 62% were with blunt trauma, 38% with penetrating. There was no difference in 28-day survival-HSD: 74.5% (0.1; 95% confidence interval [CI], -7.5 to 7.8); HS: 73.0% (-1.4; 95% CI, -8.7-6.0); and NS: 74.4%, P = 0.91. There was a higher mortality for the postrandomization subgroup of patients who did not receive blood transfusions in the first 24 hours, who received hypertonic fluids compared to NS [28-day mortality-HSD: 10% (5.2; 95% CI, 0.4-10.1); HS: 12.2% (7.4; 95% CI, 2.5-12.2); and NS: 4.8%, P < 0.01].
Conclusion: Among injured patients with hypovolemic shock, initial resuscitation fluid treatment with either HS or HSD compared with NS, did not result in superior 28-day survival. However, interpretation of these findings is limited by the early stopping of the trial.
Clinical trial registration: Clinical Trials.gov, NCT00316017.
Figures

Comment in
-
The hypertonic saline trial: a possible downside to the gold standard of double blinding.Ann Surg. 2011 Mar;253(3):442-3. doi: 10.1097/SLA.0b013e31820d32d0. Ann Surg. 2011. PMID: 21239984 No abstract available.
References
-
- Angle N, Hoyt DB, Coimbra R, et al. Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock. 1998;9(3):164–170. - PubMed
-
- Corso CO, Okamoto S, Ruttinger D, et al. Hypertonic saline dextran attenuates leukocyte accumulation in the liver after hemorrhagic shock and resuscitation. J Trauma. 1999;46(3):417–423. - PubMed
-
- Deitch EA, Shi HP, Feketeova E, et al. Hypertonic saline resuscitation limits neutrophil activation after trauma-hemorrhagic shock. Shock. 2003;19(4):328–333. - PubMed
-
- Kramer GC, Perron PR, Lindsey DC, et al. Small-volume resuscitation with hypertonic saline dextran solution. Surgery. 1986;100(2):239–247. - PubMed
-
- Smith GJ, Kramer GC, Perron P, et al. A comparison of several hypertonic solutions for resuscitation of bled sheep. J Surg Res. 1985;39(6):517–528. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL077866/HL/NHLBI NIH HHS/United States
- U01 HL077871/HL/NHLBI NIH HHS/United States
- HL077867/HL/NHLBI NIH HHS/United States
- 5U01 HL077863/HL/NHLBI NIH HHS/United States
- HL077887/HL/NHLBI NIH HHS/United States
- U01 HL077881/HL/NHLBI NIH HHS/United States
- HL077873/HL/NHLBI NIH HHS/United States
- HL077881/HL/NHLBI NIH HHS/United States
- HL077908/HL/NHLBI NIH HHS/United States
- U01 HL077887/HL/NHLBI NIH HHS/United States
- U01 HL077885/HL/NHLBI NIH HHS/United States
- CAPMC/ CIHR/Canada
- U01 HL077865/HL/NHLBI NIH HHS/United States
- U01 HL077863/HL/NHLBI NIH HHS/United States
- U01 HL077908/HL/NHLBI NIH HHS/United States
- HL077872/HL/NHLBI NIH HHS/United States
- HL077866/HL/NHLBI NIH HHS/United States
- U01 HL077873/HL/NHLBI NIH HHS/United States
- U01 HL077867/HL/NHLBI NIH HHS/United States
- HL077885/HL/NHLBI NIH HHS/United States
- HL077865/HL/NHLBI NIH HHS/United States
- U01 HL077872/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

