Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation
- PMID: 21179059
- PMCID: PMC3376542
- DOI: 10.1038/nrm3029
Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation
Abstract
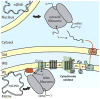
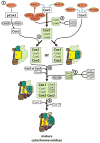
Mitochondria maintain genome and translation machinery to synthesize a small subset of subunits of the oxidative phosphorylation system. To build up functional enzymes, these organellar gene products must assemble with imported subunits that are encoded in the nucleus. New findings on the early steps of cytochrome c oxidase assembly reveal how the mitochondrial translation of its core component, cytochrome c oxidase subunit 1 (Cox1), is directly coupled to the assembly of this respiratory complex.
Figures

References
-
- Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol. 2010;11:655–667. - PubMed
-
- Vögtle FN, et al. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell. 2009;139:428–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

