Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection
- PMID: 21179070
- PMCID: PMC3130313
- DOI: 10.1038/jcbfm.2010.228
Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection
Abstract
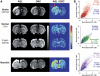
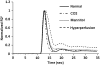
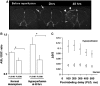
Arterial spin labeling (ASL) and dynamic susceptibility contrast (DSC) magnetic resonance imaging (MRI) are widely used to image cerebral blood flow (CBF) in stroke. This study examined how changes in tissue spin-lattice relaxation-time constant (T(1)), blood-brain barrier (BBB) permeability, and transit time affect CBF quantification by ASL and DSC in postischemic hyperperfusion in the same animals. In Group I (n=6), embolic stroke rats imaged 48 hours after stroke showed regional hyperperfusion. In normal pixels, ASL- and DSC-CBF linearly correlated pixel-by-pixel. In hyperperfusion pixels, ASL-CBF was significantly higher than DSC-CBF pixel-by-pixel (by 25%). T(1) increased from 1.76±0.14 seconds in normal pixels to 1.93±0.17 seconds in hyperperfusion pixels. Arterial transit time decreased from 300 milliseconds in normal pixels to 200 milliseconds in hyperperfusion pixels. ΔR(2)(*) profiles showed contrast-agent leakages in the hyperperfusion regions. In Group II (n=3) in which hypercapnic inhalation was used to increase CBF without BBB disruption, CBF increased overall but ASL- and DSC-CBF remained linearly correlated. In Group III (n=3) in which mannitol was used to break the BBB, ASL-CBF was significantly higher than DSC-CBF. We concluded that in normal tissue, ASL and DSC provide comparable quantitative CBF, whereas in postischemic hyperperfusion, ASL-CBF and DSC-CBF differed significantly because ischemia-induced changes in T(1) and BBB permeability affected the two methods differently.
Figures

References
-
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. - PubMed
-
- Calamante F, Gadian DG, Connelly A. Delay and dispersion effects in dynamic susceptibility contrast MRI: simulations using singular value decomposition. Magn Reson Med. 2000;44:466–473. - PubMed
-
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. - PubMed
-
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. - PubMed
-
- Donahue KM, Krouwer HG, Rand SD, Pathak AP, Marszalkowski CS, Censky SC, Prost RW. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43:845–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

