Imaging techniques for assaying lymphocyte activation in action
- PMID: 21179118
- PMCID: PMC3403683
- DOI: 10.1038/nri2903
Imaging techniques for assaying lymphocyte activation in action
Abstract
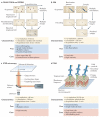
Imaging techniques have greatly improved our understanding of lymphocyte activation. Technical advances in spatial and temporal resolution and new labelling tools have enabled researchers to directly observe the activation process. Consequently, research using imaging approaches to study lymphocyte activation has expanded, providing an unprecedented level of cellular and molecular detail in the field. As a result, certain models of lymphocyte activation have been verified, others have been revised and yet others have been replaced with new concepts. In this article, we review the current imaging techniques that are used to assess lymphocyte activation in different contexts, from whole animals to single molecules, and discuss the advantages and potential limitations of these methods.
Figures




References
-
- Huse M. The T-cell-receptor signaling network. J. Cell Sci. 2009;122:1269–1273. - PubMed
-
- Lin J, Weiss A. T cell receptor signalling. J. Cell Sci. 2001;114:243–244. - PubMed
-
- Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 2002;20:371–394. - PubMed
-
- Schwartzberg PL. Genetic approaches to tyrosine kinase signaling pathways in the immune system. Immunol. Res. 2003;27:481–488. - PubMed
-
- Kaufmann SH. Immunology’s foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nature Immunol. 2008;9:705–712. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

