Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
- PMID: 21179170
- PMCID: PMC3079577
- DOI: 10.1038/nature09581
Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
Abstract
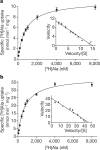
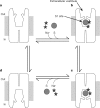
Neurotransmitter/sodium symporters (NSSs) couple the uptake of neurotransmitter with one or more sodium ions, removing neurotransmitter from the synaptic cleft. NSSs are essential to the function of chemical synapses, are associated with multiple neurological diseases and disorders, and are the targets of therapeutic and illicit drugs. LeuT, a prokaryotic orthologue of the NSS family, is a model transporter for understanding the relationships between molecular mechanism and atomic structure in a broad range of sodium-dependent and sodium-independent secondary transporters. At present there is a controversy over whether there are one or two high-affinity substrate binding sites in LeuT. The first-reported crystal structure of LeuT, together with subsequent functional and structural studies, provided direct evidence for a single, high-affinity, centrally located substrate-binding site, defined as the S1 site. Recent binding, flux and molecular simulation studies, however, have been interpreted in terms of a model where there are two high-affinity binding sites: the central, S1, site and a second, the S2 site, located within the extracellular vestibule. Furthermore, it was proposed that the S1 and S2 sites are allosterically coupled such that occupancy of the S2 site is required for the cytoplasmic release of substrate from the S1 site. Here we address this controversy by performing direct measurement of substrate binding to wild-type LeuT and to S2 site mutants using isothermal titration calorimetry, equilibrium dialysis and scintillation proximity assays. In addition, we perform uptake experiments to determine whether the proposed allosteric coupling between the putative S2 site and the S1 site manifests itself in the kinetics of substrate flux. We conclude that LeuT harbours a single, centrally located, high-affinity substrate-binding site and that transport is well described by a simple, single-substrate kinetic mechanism.
Figures


References
-
- Sobczak I, Lolkema JS. Structural and mechanistic diversity of secondary transporters. Curr. Opin. Microbiol. 2005;8:161–167. - PubMed
-
- Hahn MK, Blakely RD. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. Pharmacogenomics J. 2002;2:217–235. - PubMed
-
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

