Preparation and characterization of microporous poly(D,L-lactic acid) film for tissue engineering scaffold
- PMID: 21179227
- PMCID: PMC3000204
- DOI: 10.2147/IJN.S13169
Preparation and characterization of microporous poly(D,L-lactic acid) film for tissue engineering scaffold
Abstract
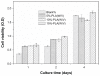
We prepared a series of microporous films based on poly(d,l-lactic acid) (PLA) via phase separation. According to scanning electron microscopy (SEM), a 3-dimensional foamy structure with multimicrometer scale pores on the air surface of film could be observed. As the morphology of PLA film could not be stabilized using solvent-nonsolvent phase separation, we investigated the effect of temperature, air movement, and concentration on the properties of microporous PLA films. The results show that when the temperature was 25°C in a vacuum, it was easy to prepare PLA film with micropores, and it was stable. As the relationship between the morphology and formation factors was clear and the morphology of the PLA film was controllable, we studied the PLA film's potential use for cell culture. SEM results showed that NIH3T3 cell could be adhered on the surface of film well after incubation for 2 days. Meanwhile, in vitro culture experiments revealed the great biocompatibility of the scaffold for adsorption and proliferation of fibroblasts.
Keywords: cell culture; phase separation; poly(d,l-lactic acid); scaffold.
Figures







References
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. - PubMed
-
- Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. - PubMed
-
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. - PubMed
-
- You Y, Min BM, Lee SJ, Lee TS, Park WH. In vitro degradation behavior of electrospun polyglycolide, polylactide, and poly (lactide-co-glycolide) J Appl Polym Sci. 2005;95(2):193–200.
-
- Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6(11):908–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

