Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium
- PMID: 21179238
- PMCID: PMC3002964
Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium
Abstract
Purpose: To determine whether epithelial-mesenchymal transition is involved in the development of corneal subepithelial fibrosis (pannus).
Methods: Frozen samples of pannus tissue removed from human corneas with a diagnosis of total limbal stem cell deficiency were characterized by immunostaining for both epithelial and mesenchymal markers. We selected transformation-related protein 63 (p63) and pancytokeratin as epithelial markers and vimentin and α-smooth muscle actin (α-SMA) as mesenchymal markers. Immunostaining for β-catenin and E-cadherin was performed to determine wingless-Int (Wnt)-pathway activation. RT-PCR analysis was also performed on epithelial tissue obtained from pannus samples after dispase digestion.
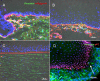
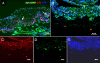
Results: Immunohistochemistry revealed strong nuclear expression of p63 and weak intercellular expression of E-cadherin in epithelial basal cells of pannus tissue. Furthermore, translocation of β-catenin from intercellular junctions to the nucleus and cytoplasm was also observed. Double-positive cells for both p63 and α-SMA were observed in the subepithelial stroma of pannus tissue, which was supported by RT-PCR and cytospin analysis.
Conclusions: Epithelial-mesenchymal transition may be partially involved in the development of subepithelial corneal fibrosis due to total limbal stem cell deficiency.
Figures





References
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. - PubMed
-
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–23. - PubMed
-
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. - PubMed
-
- Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
