Iota-carrageenan is a potent inhibitor of influenza A virus infection
- PMID: 21179403
- PMCID: PMC3001860
- DOI: 10.1371/journal.pone.0014320
Iota-carrageenan is a potent inhibitor of influenza A virus infection
Abstract
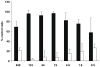
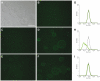
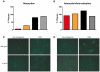
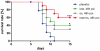
The 2009 flu pandemic and the appearance of oseltamivir-resistant H1N1 influenza strains highlight the need for treatment alternatives. One such option is the creation of a protective physical barrier in the nasal cavity. In vitro tests demonstrated that iota-carrageenan is a potent inhibitor of influenza A virus infection, most importantly also of pandemic H1N1/2009 in vitro. Consequently, we tested a commercially available nasal spray containing iota-carrageenan in an influenza A mouse infection model. Treatment of mice infected with a lethal dose of influenza A PR8/34 H1N1 virus with iota-carrageenan starting up to 48 hours post infection resulted in a strong protection of mice similar to mice treated with oseltamivir. Since alternative treatment options for influenza are rare, we conclude that the nasal spray containing iota-carrageenan is an alternative to neuraminidase inhibitors and should be tested for prevention and treatment of influenza A in clinical trials in humans.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

