Fixed-dose Sumatriptan/Naproxen Sodium Compared with each Monotherapy Utilizing the Novel Composite Endpoint of Sustained Pain-free/no Adverse Events
- PMID: 21179523
- PMCID: PMC3002629
- DOI: 10.1177/1756285609102769
Fixed-dose Sumatriptan/Naproxen Sodium Compared with each Monotherapy Utilizing the Novel Composite Endpoint of Sustained Pain-free/no Adverse Events
Abstract
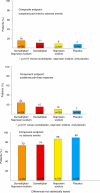
A novel composite endpoint, sustained pain-free/no adverse events, was recently proposed as a more rigorous means of capturing in a single measure the attributes of migraine pharmacotherapy that patients consider most important: rapid and sustained pain-free response with no side-effects. Using pooled data from two replicate randomized, double-blind, parallel-group, placebo-controlled studies, this post hoc analysis compared the fixed-dose combination tablet sumatriptan/naproxen sodium (n = 726) with sumatriptan monotherapy (n = 723), naproxen sodium monotherapy (n = 720), and placebo (n = 742) with respect to sustained pain-free/no adverse events and closely related composite measures. Sustained pain-free/no adverse events was defined as having both a sustained pain-free response from 2 through 24 hours post-dose with no use of rescue medication and having no adverse events within up to 5 days after dosing with study medication. The percentage of patients with sustained pain-free/no adverse events was 16% with sumatriptan/naproxen sodium compared with 11%, 9% and 7% for sumatriptan, naproxen sodium and placebo, respectively (p<0.01 sumatriptan/naproxen sodium versus each other treatment). Sumatriptan/naproxen sodium was also significantly more effective than sumatriptan, naproxen sodium, and placebo for other composite endpoints including the percentages of patients with (1) sustained pain-free/no adverse events within 1 day; (2) sustained pain-free/no drug-related adverse events within up to 5 days; (3) sustained pain-free/no drug-related adverse events within 1 day; (4) sustained pain relief/no adverse events within up to 5 days; and (5) sustained pain relief/no adverse events within 1 day. The results demonstrate the superiority of sumatriptan/naproxen sodium to sumatriptan monotherapy, naproxen sodium monotherapy and placebo with respect to the rigorous and clinically relevant endpoint of sustained pain-free/no adverse events and reinforce the usefulness of utilizing this new composite endpoint.
Keywords: efficacy; headache; migraine; safety; sumatriptan/naproxen sodium; sustained pain-free; tolerability.
Figures
References
-
- Brandes J.L., Kudrow D., Cady R., Tiseo P.J., Sun W., Sikes C.R. (2005) Eletriptan in the early treatment of acute migraine: influence of pain intensity and time of dosing. Cephalalgia 25: 735–742 - PubMed
-
- Carpay J., Schoenen J., Ahmad F., Kinrade F., Boswell D. (2004) Efficacy and tolerability of sumatriptan tablets in a fast-disintegrating, rapidrelease formulation for the acute treatment of migraine: results of a multicenter, randomized, placebo-controlled study. Clin Ther 26: 214–223 - PubMed
-
- Dodick D.W., Sandrini G., Williams P. (2007) Use of the sustained pain-free plus no adverse events endpoint in clinical trials of triptans in acute migraine. CNS Drugs 21: 73–82 - PubMed
LinkOut - more resources
Full Text Sources