Grifonin-1: a small HIV-1 entry inhibitor derived from the algal lectin, Griffithsin
- PMID: 21179548
- PMCID: PMC3002932
- DOI: 10.1371/journal.pone.0014360
Grifonin-1: a small HIV-1 entry inhibitor derived from the algal lectin, Griffithsin
Abstract
Background: Griffithsin, a 121-residue protein isolated from a red algal Griffithsia sp., binds high mannose N-linked glycans of virus surface glycoproteins with extremely high affinity, a property that allows it to prevent the entry of primary isolates and laboratory strains of T- and M-tropic HIV-1. We used the sequence of a portion of griffithsin's sequence as a design template to create smaller peptides with antiviral and carbohydrate-binding properties.
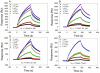
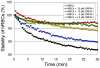
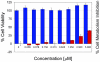
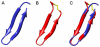
Methodology/results: The new peptides derived from a trio of homologous β-sheet repeats that comprise the motifs responsible for its biological activity. Our most active antiviral peptide, grifonin-1 (GRFN-1), had an EC50 of 190.8±11.0 nM in in vitro TZM-bl assays and an EC(50) of 546.6±66.1 nM in p24gag antigen release assays. GRFN-1 showed considerable structural plasticity, assuming different conformations in solvents that differed in polarity and hydrophobicity. Higher concentrations of GRFN-1 formed oligomers, based on intermolecular β-sheet interactions. Like its parent protein, GRFN-1 bound viral glycoproteins gp41 and gp120 via the N-linked glycans on their surface.
Conclusion: Its substantial antiviral activity and low toxicity in vitro suggest that GRFN-1 and/or its derivatives may have therapeutic potential as topical and/or systemic agents directed against HIV-1.
Conflict of interest statement
Figures








References
-
- Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. - PubMed
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. - PubMed
-
- Haggerty CM, Pitt E, Siliciano RF. The latent reservoir for HIV-1 in resting CD4+ T cells and other viral reservoirs during chronic infection: insights from treatment and treatment-interruption trials. Curr Opin HIV AIDS. 2006;1:62–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

