Food and drug administration's critical path initiative and innovations in drug development paradigm: Challenges, progress, and controversies
- PMID: 21180462
- PMCID: PMC2996064
- DOI: 10.4103/0975-7406.72130
Food and drug administration's critical path initiative and innovations in drug development paradigm: Challenges, progress, and controversies
Abstract
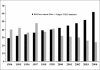
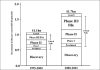
During the last decade, despite increased investment in drug research and development related activity, stagnation in new drug discovery has been documented. Despite a 70% increase in investment in research and development-related activities, a 40% fall in launch of new chemical entities was seen during 1994-2004. A steep rise in the attrition rate of drug development has complicated the matter. Rising cost and increased attrition rates proved major barriers to investment in higher risk drugs or in therapies for uncommon diseases or diseases that predominantly afflict the poor. This prompted Food and Drug Administration (FDA) to highlight this problem in a 2004 white paper classified as "Critical Path Initiative" (CPI) and to initiate steps to target stagnation and rise in attrition rates. Many new drug development projects have started worldwide taking cue from CPI; adopting microdosing, adaptive designs and taking advantage of newly developed biomarkers under the CPI. This review discusses the various strategies adopted under CPI to decrease attrition rate and stagnation of new drug development, and the challenges and controversies associated with CPI.
Keywords: Biomarkers; clinical trials; drug development; microdosing.
Conflict of interest statement
Figures
Similar articles
-
Adaptive design clinical trials: Methodology, challenges and prospect.Indian J Pharmacol. 2010 Aug;42(4):201-7. doi: 10.4103/0253-7613.68417. Indian J Pharmacol. 2010. PMID: 20927243 Free PMC article.
-
Phase 0 - Microdosing strategy in clinical trials.Indian J Pharmacol. 2008 Nov;40(6):240-2. doi: 10.4103/0253-7613.45147. Indian J Pharmacol. 2008. PMID: 21279177 Free PMC article.
-
Adaptive designs in clinical trials.Perspect Clin Res. 2011 Jan;2(1):23-7. doi: 10.4103/2229-3485.76286. Perspect Clin Res. 2011. PMID: 21584178 Free PMC article.
-
Paving the critical path: how can clinical pharmacology help achieve the vision?Clin Pharmacol Ther. 2007 Feb;81(2):170-7. doi: 10.1038/sj.clpt.6100045. Clin Pharmacol Ther. 2007. PMID: 17259944 Review.
-
Emerging Safety Science: Workshop Summary.Washington (DC): National Academies Press (US); 2008. Washington (DC): National Academies Press (US); 2008. PMID: 20669415 Free Books & Documents. Review.
Cited by
-
Attitudes and opinions regarding confirmatory adaptive clinical trials: a mixed methods analysis from the Adaptive Designs Accelerating Promising Trials into Treatments (ADAPT-IT) project.Trials. 2016 Jul 29;17:373. doi: 10.1186/s13063-016-1493-z. Trials. 2016. PMID: 27473126 Free PMC article.
-
Visualizing Extracellular Vesicles and Their Function in 3D Tumor Microenvironment Models.Int J Mol Sci. 2021 Apr 30;22(9):4784. doi: 10.3390/ijms22094784. Int J Mol Sci. 2021. PMID: 33946403 Free PMC article. Review.
-
DeepLPI: a novel deep learning-based model for protein-ligand interaction prediction for drug repurposing.Sci Rep. 2022 Oct 28;12(1):18200. doi: 10.1038/s41598-022-23014-1. Sci Rep. 2022. PMID: 36307509 Free PMC article.
-
An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors.Sci Transl Med. 2015 Apr 22;7(284):284ra57. doi: 10.1126/scitranslmed.3010564. Sci Transl Med. 2015. PMID: 25904741 Free PMC article.
-
A novel marker procalcitonin may help stem the antibiotic overuse in emergency setting.Int J Appl Basic Med Res. 2013 Jul;3(2):77-83. doi: 10.4103/2229-516X.117051. Int J Appl Basic Med Res. 2013. PMID: 24083140 Free PMC article.
References
-
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: New estimates of drug development costs. J Health Econ. 2003;22:151–85. - PubMed
-
- Adams CP, Brantner VV. Spending on new drug development. Health Econ. 2010;19:130–41. - PubMed
-
- Oates JA. The science of drug therapy. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s The pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill; 2006. p. 134.
-
- Innovation or Stagnation: Challenge and opportunity on the critical path to new medical products. Washington DC, USA: Food and Drug Administration; 2004. Food and Drug Administration.
-
- Berkowitz BA. Development and Regulation of Drugs. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 11th ed. New Delhi: Tata McGraw Hill; 2009. p. 67.