C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling
- PMID: 21183071
- PMCID: PMC3035164
- DOI: 10.1016/j.cell.2010.11.036
C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling
Abstract
The heart has the ability to grow in size in response to exercise, but little is known about the transcriptional mechanisms underlying physiological hypertrophy. Adult cardiomyocytes have also recently been proven to hold the potential for proliferation, a process that could be of great importance for regenerative medicine. Using a unique RT-PCR-based screen against all transcriptional components, we showed that C/EBPβ was downregulated with exercise, whereas the expression of CITED4 was increased. Reduction of C/EBPβ in vitro and in vivo resulted in a phenocopy of endurance exercise with cardiomyocyte hypertrophy and proliferation. This proliferation was mediated, at least in part, by the increased CITED4. Importantly, mice with reduced cardiac C/EBPβ levels displayed substantial resistance to cardiac failure upon pressure overload. These data indicate that C/EBPβ represses cardiomyocyte growth and proliferation in the adult mammalian heart and that reduction in C/EBPβ is a central signal in physiologic hypertrophy and proliferation.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
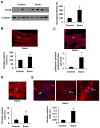

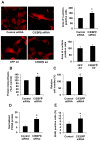

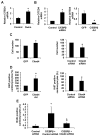

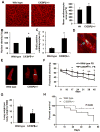
Comment in
-
Heart-healthy hypertrophy.Cell Metab. 2011 Jan 5;13(1):3-4. doi: 10.1016/j.cmet.2010.12.012. Cell Metab. 2011. PMID: 21195341 Free PMC article.
References
-
- Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res. 2003;92:1079–1088. - PubMed
-
- Aoyama T, Matsui T, Novikov M, Park J, Hemmings B, Rosenzweig A. Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 2005;111:1652–1659. - PubMed
-
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

