Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals
- PMID: 21183072
- PMCID: PMC3039484
- DOI: 10.1016/j.cell.2010.12.008
Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals
Abstract
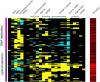
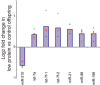
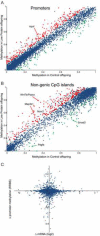
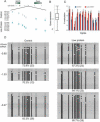
Epigenetic information can be inherited through the mammalian germline and represents a plausible transgenerational carrier of environmental information. To test whether transgenerational inheritance of environmental information occurs in mammals, we carried out an expression profiling screen for genes in mice that responded to paternal diet. Offspring of males fed a low-protein diet exhibited elevated hepatic expression of many genes involved in lipid and cholesterol biosynthesis and decreased levels of cholesterol esters, relative to the offspring of males fed a control diet. Epigenomic profiling of offspring livers revealed numerous modest (∼20%) changes in cytosine methylation depending on paternal diet, including reproducible changes in methylation over a likely enhancer for the key lipid regulator Ppara. These results, in conjunction with recent human epidemiological data, indicate that parental diet can affect cholesterol and lipid metabolism in offspring and define a model system to study environmental reprogramming of the heritable epigenome.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Epigenetics: Dad's diet lives on.Nat Rev Genet. 2011 Feb;12(2):80. doi: 10.1038/nrg2941. Nat Rev Genet. 2011. PMID: 21245827 No abstract available.
-
You are what your dad ate.Cell Metab. 2011 Feb 2;13(2):115-7. doi: 10.1016/j.cmet.2011.01.011. Cell Metab. 2011. PMID: 21284975
References
-
- Anderson LM, Riffle L, Wilson R, Travlos GS, Lubomirski MS, Alvord WG. Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition (Burbank, Los Angeles County, Calif. 2006;22:327–331. - PubMed
-
- Avital E, Jablonka E. Animal traditions : behavioural inheritance in evolution. Cambridge University Press; Cambridge, UK: New York: 2000. - PubMed
-
- Bartolomei MS, Webber AL, Brunkow ME, Tilghman SM. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes Dev. 1993;7:1663–1673. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

