Regulation of peptidoglycan synthesis by outer-membrane proteins
- PMID: 21183073
- PMCID: PMC3060616
- DOI: 10.1016/j.cell.2010.11.038
Regulation of peptidoglycan synthesis by outer-membrane proteins
Abstract
Growth of the mesh-like peptidoglycan (PG) sacculus located between the bacterial inner and outer membranes (OM) is tightly regulated to ensure cellular integrity, maintain cell shape, and orchestrate division. Cytoskeletal elements direct placement and activity of PG synthases from inside the cell, but precise spatiotemporal control over this process is poorly understood. We demonstrate that PG synthases are also controlled from outside of the sacculus. Two OM lipoproteins, LpoA and LpoB, are essential for the function, respectively, of PBP1A and PBP1B, the major E. coli bifunctional PG synthases. Each Lpo protein binds specifically to its cognate PBP and stimulates its transpeptidase activity, thereby facilitating attachment of new PG to the sacculus. LpoB shows partial septal localization, and our data suggest that the LpoB-PBP1B complex contributes to OM constriction during cell division. LpoA/LpoB and their PBP-docking regions are restricted to γ-proteobacteria, providing models for niche-specific regulation of sacculus growth.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


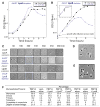
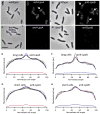
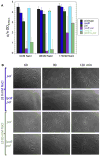

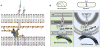
Comment in
-
New ways to make old walls: bacterial surprises.Cell. 2010 Dec 23;143(7):1042-4. doi: 10.1016/j.cell.2010.12.011. Cell. 2010. PMID: 21183069
References
-
- Aaron M, Charbon G, Lam H, Schwarz H, Vollmer W, Jacobs-Wagner C. The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol Microbiol. 2007;64:938–952. - PubMed
-
- Aarsman ME, Piette A, Fraipont C, Vinkenvleugel TM, Nguyen-Distèche M, den Blaauwen T. Maturation of the Escherichia coli divisome occurs in two steps. Mol Microbiol. 2005;55:1631–1645. - PubMed
-
- Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–653. - PubMed
-
- Bertsche U, Breukink E, Kast T, Vollmer W. In vitro murein peptidoglycan synthesis by dimers of the bifunctional transglycosylase-transpeptidase PBP1B from Escherichia coli. J Biol Chem. 2005;280:38096–38101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

