Conjugation polymer nanobelts: a novel fluorescent sensing platform for nucleic acid detection
- PMID: 21183465
- PMCID: PMC3064799
- DOI: 10.1093/nar/gkq1294
Conjugation polymer nanobelts: a novel fluorescent sensing platform for nucleic acid detection
Abstract
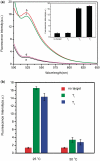
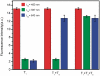
In this article, we report on the facile and rapid synthesis of conjugation polymer poly(p-phenylenediamine) nanobelts (PNs) via room temperature chemical oxidation polymerization of p-phenylenediamine monomers by ammonium persulfate in aqueous medium. We further demonstrate the proof-of-concept that PNs can be used as an effective fluorescent sensing platform for nucleic acid detection for the first time. The general concept used in this approach lies in the facts that the adsorption of the fluorescently labeled single-stranded DNA probe by PN leads to substantial fluorescence quenching, followed by specific hybridization with the complementary region of the target DNA sequence. This results in desorption of the hybridized complex from PN surface and subsequent recovery of fluorescence. We also show that the sensing platform described herein can be used for multiplexing detection of nucleic acid sequences.
Figures





References
-
- Gresham D, Ruderfer DM, Pratt SC, Schacherer J, Dunham MJ, Botstein D, Kruglyak L. Genome-wide detection of polymorphisms at nucleotide resolution with a single DNA microarray. Science. 2006;311:1932–1936. - PubMed
-
- Sassolas A, Leca-Bouvier BD, Blum LJ. DNA biosensors and microarrays. Chem. Rev. 2008;108:109–139. - PubMed
-
- Didenko VV, editor. Fluorescent Energy Transfer Nucleic Acid Probes: designs and Protocols. Totowa, NJ: Human Press; 2006.
-
- Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 1996;14:303–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

