Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway
- PMID: 21183792
- PMCID: PMC3007144
- DOI: 10.1172/JCI42874
Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway
Abstract
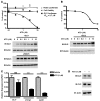
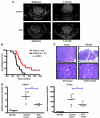
The Hedgehog (Hh) pathway is activated in some human cancers, including medulloblastoma. The glioma-associated oncogene homolog (GLI) transcription factors are critical mediators of the activated Hh pathway, and their expression may be elevated in some tumors independent of upstream Hh signaling. Thus, therapies targeting GLI transcription factors may benefit a wide spectrum of patients with mutations at different nodal points of the Hh pathway. In this study, we present evidence that arsenic trioxide (ATO) suppresses human cancer cell growth and tumor development in mice by inhibiting GLI1. Mechanistically, ATO directly bound to GLI1 protein, inhibited its transcriptional activity, and decreased expression of endogenous GLI target genes. Consistent with this, ATO inhibited the growth of human cancer cell lines that depended on upregulated GLI expression in vitro and in vivo in a xenograft model of Ewing sarcoma. Furthermore, ATO improved survival of a clinically relevant spontaneous mouse model of medulloblastoma with activated Hh pathway signaling. Our results establish ATO as a Hh pathway inhibitor acting at the level of GLI1 both in vitro and in vivo. These results warrant the clinical investigation of ATO for tumors with activated Hh/GLI signaling, in particular patients who develop resistance to current therapies targeting the Hh pathway upstream of GLI.
Figures





Comment in
-
Arsenic: a potentially useful poison for Hedgehog-driven cancers.J Clin Invest. 2011 Jan;121(1):14-6. doi: 10.1172/JCI45692. Epub 2010 Dec 22. J Clin Invest. 2011. PMID: 21183780 Free PMC article.
References
-
- Pietsch T, et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57(11):2085–2088. - PubMed
-
- Reifenberger J, et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58(9):1798–1803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

