Hypothermia postpones DNA damage repair in irradiated cells and protects against cell killing
- PMID: 21185842
- PMCID: PMC6944433
- DOI: 10.1016/j.mrfmmm.2010.12.006
Hypothermia postpones DNA damage repair in irradiated cells and protects against cell killing
Abstract
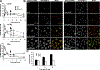
Hibernation is an established strategy used by some homeothermic organisms to survive cold environments. In true hibernation, the core body temperature of an animal may drop to below 0°C and metabolic activity almost cease. The phenomenon of hibernation in humans is receiving renewed interest since several cases of victims exhibiting core body temperatures as low as 13.7°C have been revived with minimal lasting deficits. In addition, local cooling during radiotherapy has resulted in normal tissue protection. The experiments described in this paper were prompted by the results of a very limited pilot study, which showed a suppressed DNA repair response of mouse lymphocytes collected from animals subjected to 7-Gy total body irradiation under hypothermic (13°C) conditions, compared to normothermic controls. Here we report that human BJ-hTERT cells exhibited a pronounced radioprotective effect on clonogenic survival when cooled to 13°C during and 12h after irradiation. Mild hypothermia at 20 and 30°C also resulted in some radioprotection. The neutral comet assay revealed an apparent lack on double strand break (DSB) rejoining at 13°C. Extension of the mouse lymphocyte study to ex vivo-irradiated human lymphocytes confirmed lower levels of induced phosphorylated H2AX (γ-H2AX) and persistence of the lesions at hypothermia compared to the normal temperature. Parallel studies of radiation-induced oxidatively clustered DNA lesions (OCDLs) revealed partial repair at 13°C compared to the rapid repair at 37°C. For both γ-H2AX foci and OCDLs, the return of lymphocytes to 37°C resulted in the resumption of normal repair kinetics. These results, as well as observations made by others and reviewed in this study, have implications for understanding the radiobiology and protective mechanisms underlying hypothermia and potential opportunities for exploitation in terms of protecting normal tissues against radiation.
2011. Published by Elsevier B.V.
Conflict of interest statement
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

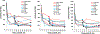
References
-
- Morgan G, Ward R, Barton M, The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies, Clin. Oncol. (R. Coll. Radiol.) 16 (2004) 549–560. - PubMed
-
- Jain S, Agarwal J, Laskar S, Gupta T, Shrivastava S, Radiation recall dermatitis with gatifloxacin: a review of literature, J. Med. Imaging Radiat. Oncol 52 (2008) 191–193. - PubMed
-
- Mols F, van den Hurk CJ, Vingerhoets AJ, Breed WP, Scalp cooling to prevent chemotherapy-induced hair loss: practical and clinical considerations, Support Care Cancer 17 (2009) 181–189. - PubMed
-
- Cucinotta FA, Durante M, Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings, Lancet Oncol. 7 (2006) 431–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

