Repeated, intermittent exposures to diisopropylfluorophosphate in rats: protracted effects on cholinergic markers, nerve growth factor-related proteins, and cognitive function
- PMID: 21185910
- PMCID: PMC3040272
- DOI: 10.1016/j.neuroscience.2010.12.031
Repeated, intermittent exposures to diisopropylfluorophosphate in rats: protracted effects on cholinergic markers, nerve growth factor-related proteins, and cognitive function
Abstract
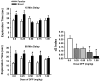
Organophosphates (OPs) pose a constant threat to human health due to their widespread use as pesticides and their potential employment in military and terrorist attacks. The acute toxicity of OPs has been extensively studied; however, the consequences of prolonged or repeated exposure to levels of OPs that produce no overt signs of acute toxicity (i.e. subthreshold levels) are poorly understood. Further, there is clinical evidence that such repeated exposures to OPs lead to prolonged deficits in cognition, although the mechanism for this effect is unknown. In this study, the behavioral and neurochemical effects of repeated, intermittent, and subthreshold exposures to the alkyl OP, diisopropylfluorophosphate (DFP) were investigated. Rats were injected with DFP s.c. (dose range, 0.25-1.0 mg/kg) every other day over the course of 30 days, and then given a 2 week, DFP-free washout period. In behavioral experiments conducted at various times during the washout period, dose dependent decrements in a water maze hidden platform task and a spontaneous novel object recognition (NOR) procedure were observed, while prepulse inhibition of the acoustic startle response was unaffected. There were modest decreases in open field locomotor activity and grip strength (particularly during the DFP exposure period); however, rotarod performance and water maze swim speeds were not affected. After washout, DFP concentrations were minimal in plasma and brain, however, cholinesterase inhibition was still detectable in the brain. Moreover, the 1.0 mg/kg dose of DFP was associated with (brain region-dependent) alterations in nerve growth factor-related proteins and cholinergic markers. The results of this prospective animal study thus provide evidence to support two novel hypotheses: (1) that intermittent, subthreshold exposures to alkyl OPs can lead to protracted deficits in specific domains of cognition and (2) that such cognitive deficits may be related to persistent functional changes in brain neurotrophin and cholinergic pathways.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures









References
-
- Barnaby F. The nuclear arsenals and nuclear disarmament. Med Confl Surviv. 1998;14(4):314–20. - PubMed
-
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. - PubMed
-
- Bartolini L, Casamenti F, Pepeu G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol Biochem Behav. 1996;53:277–283. - PubMed
-
- Black RM, Clarke RJ, Read RW, Reid MT. Application of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry to the analysis of chemical warfare samples, found to contain certain residues of the nerve agent sarin, sulphur mustard and their degradation products. J Chromatogr A. 1994;662(2):301–21. - PubMed
-
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

