NFAT regulates pre-synaptic development and activity-dependent plasticity in Drosophila
- PMID: 21185939
- PMCID: PMC3030698
- DOI: 10.1016/j.mcn.2010.12.010
NFAT regulates pre-synaptic development and activity-dependent plasticity in Drosophila
Abstract
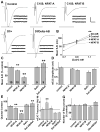
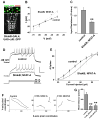
The calcium-regulated transcription factor NFAT is emerging as a key regulator of neuronal development and plasticity but precise cellular consequences of NFAT function remain poorly understood. Here, we report that the single Drosophila NFAT homolog is widely expressed in the nervous system including motor neurons and unexpectedly controls neural excitability. Likely due to this effect on excitability, NFAT regulates overall larval locomotion and both chronic and acute forms of activity-dependent plasticity at the larval glutamatergic neuro-muscular synapse. Specifically, NFAT-dependent synaptic phenotypes include changes in the number of pre-synaptic boutons, stable modifications in synaptic microtubule architecture and pre-synaptic transmitter release, while no evidence is found for synaptic retraction or alterations in the level of the synaptic cell adhesion molecule FasII. We propose that NFAT regulates pre-synaptic development and constrains long-term plasticity by dampening neuronal excitability.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





References
-
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Brunner A, O’Kane CJ. The fascination of the Drosophila NMJ. Trends Genet. 1997;13:85–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

