Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits
- PMID: 21186265
- PMCID: PMC3009842
- DOI: 10.1093/brain/awq341
Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits
Abstract
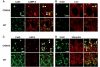
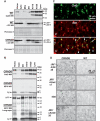
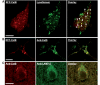
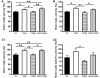
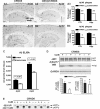
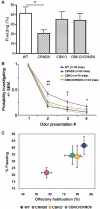
Autophagy, a major degradative pathway for proteins and organelles, is essential for survival of mature neurons. Extensive autophagic-lysosomal pathology in Alzheimer's disease brain contributes to Alzheimer's disease pathogenesis, although the underlying mechanisms are not well understood. Here, we identified and characterized marked intraneuronal amyloid-β peptide/amyloid and lysosomal system pathology in the Alzheimer's disease mouse model TgCRND8 similar to that previously described in Alzheimer's disease brains. We further establish that the basis for these pathologies involves defective proteolytic clearance of neuronal autophagic substrates including amyloid-β peptide. To establish the pathogenic significance of these abnormalities, we enhanced lysosomal cathepsin activities and rates of autophagic protein turnover in TgCRND8 mice by genetically deleting cystatin B, an endogenous inhibitor of lysosomal cysteine proteases. Cystatin B deletion rescued autophagic-lysosomal pathology, reduced abnormal accumulations of amyloid-β peptide, ubiquitinated proteins and other autophagic substrates within autolysosomes/lysosomes and reduced intraneuronal amyloid-β peptide. The amelioration of lysosomal function in TgCRND8 markedly decreased extracellular amyloid deposition and total brain amyloid-β peptide 40 and 42 levels, and prevented the development of deficits of learning and memory in fear conditioning and olfactory habituation tests. Our findings support the pathogenic significance of autophagic-lysosomal dysfunction in Alzheimer's disease and indicate the potential value of restoring normal autophagy as an innovative therapeutic strategy for Alzheimer's disease.
Figures

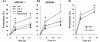


Similar articles
-
Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits.Brain. 2014 Dec;137(Pt 12):3300-18. doi: 10.1093/brain/awu278. Epub 2014 Sep 29. Brain. 2014. PMID: 25270989 Free PMC article.
-
Therapeutic effects of remediating autophagy failure in a mouse model of Alzheimer disease by enhancing lysosomal proteolysis.Autophagy. 2011 Jul;7(7):788-9. doi: 10.4161/auto.7.7.15596. Epub 2011 Jul 1. Autophagy. 2011. PMID: 21464620 Free PMC article.
-
Cyclodextrin has conflicting actions on autophagy flux in vivo in brains of normal and Alzheimer model mice.Hum Mol Genet. 2017 Mar 1;26(5):843-859. doi: 10.1093/hmg/ddx001. Hum Mol Genet. 2017. PMID: 28062666 Free PMC article.
-
Neuronal autophagy: self-eating or self-cannibalism in Alzheimer's disease.Neurochem Res. 2013 Sep;38(9):1769-73. doi: 10.1007/s11064-013-1082-4. Epub 2013 Jun 5. Neurochem Res. 2013. PMID: 23737325 Free PMC article. Review.
-
Alzheimer's disease and the autophagic-lysosomal system.Neurosci Lett. 2019 Apr 1;697:49-58. doi: 10.1016/j.neulet.2018.05.017. Epub 2018 May 18. Neurosci Lett. 2019. PMID: 29758300 Review.
Cited by
-
Autophagy impairment: a crossroad between neurodegeneration and tauopathies.BMC Biol. 2012 Sep 21;10:78. doi: 10.1186/1741-7007-10-78. BMC Biol. 2012. PMID: 22999309 Free PMC article.
-
Y682G Mutation of Amyloid Precursor Protein Promotes Endo-Lysosomal Dysfunction by Disrupting APP-SorLA Interaction.Front Cell Neurosci. 2015 Apr 7;9:109. doi: 10.3389/fncel.2015.00109. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25904844 Free PMC article.
-
Enhancing astrocytic lysosome biogenesis facilitates Aβ clearance and attenuates amyloid plaque pathogenesis.J Neurosci. 2014 Jul 16;34(29):9607-20. doi: 10.1523/JNEUROSCI.3788-13.2014. J Neurosci. 2014. PMID: 25031402 Free PMC article.
-
Unfolded Protein Response and Macroautophagy in Alzheimer's, Parkinson's and Prion Diseases.Molecules. 2015 Dec 18;20(12):22718-56. doi: 10.3390/molecules201219865. Molecules. 2015. PMID: 26694349 Free PMC article. Review.
-
Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration.Int J Mol Sci. 2023 Mar 24;24(7):6180. doi: 10.3390/ijms24076180. Int J Mol Sci. 2023. PMID: 37047151 Free PMC article. Review.
References
-
- Abrahamson M. Cystatins. Methods Enzymol. 1994;244:685–700. - PubMed
-
- Alakurtti K, Weber E, Rinne R, Theil G, de Haan GJ, Lindhout D, et al. Loss of lysosomal association of cystatin B proteins representing progressive myoclonus epilepsy, EPM1, mutations. Eur J Hum Genet. 2005;13:208–15. - PubMed
-
- Anderluh G, Gutierrez-Aguirre I, Rabzelj S, Ceru S, Kopitar-Jerala N, Macek P, et al. Interaction of human stefin B in the prefibrillar oligomeric form with membranes. Correlation with cellular toxicity. FEBS J. 2005;272:3042–51. - PubMed
-
- Auteri J, Okada A, Bochaki V, Dice J. Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J Cell Physiol. 1983;115:167–74. - PubMed
-
- Bayer TA, Wirths O. Review on the APP/PS1KI mouse model: intraneuronal Abeta accumulation triggers axonopathy, neuron loss and working memory impairment. Genes Brain Behav. 2008;7(Suppl. 1):6–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

