Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury
- PMID: 21186323
- PMCID: PMC3044993
- DOI: 10.1074/jbc.M110.187831
Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury
Abstract
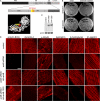
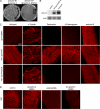
Costameres are cellular sites of mechanotransduction in heart and skeletal muscle where dystrophin and its membrane-spanning partner dystroglycan distribute intracellular contractile forces into the surrounding extracellular matrix. Resolution of a functional costamere interactome is still limited but likely to be critical for understanding forms of muscular dystrophy and cardiomyopathy. Dystrophin binds a set of membrane-associated proteins (the dystrophin-glycoprotein complex) as well as γ-actin and microtubules and also is required to align sarcolemmal microtubules with costameres. Ankyrin-B binds to dystrophin, dynactin-4, and microtubules and is required for sarcolemmal association of these proteins as well as dystroglycan. We report here that ankyrin-B interactions with β2 spectrin and dynactin-4 are required for localization of dystrophin, dystroglycan, and microtubules at costameres as well as protection of muscle from exercise-induced injury. Knockdown of dynactin-4 in adult mouse skeletal muscle phenocopied depletion of ankyrin-B and resulted in loss of sarcolemmal dystrophin, dystroglycan, and microtubules. Moreover, mutations of ankyrin-B and of dynactin-4 that selectively impaired binary interactions between these proteins resulted in loss of their costamere-localizing activity and increased muscle fiber fragility as a result of loss of costamere-associated dystrophin and dystroglycan. In addition, costamere-association of dynactin-4 did not require dystrophin but did depend on β2 spectrin and ankyrin-B, whereas costamere association of ankyrin-B required β2 spectrin. Together, these results are consistent with a functional hierarchy beginning with β2 spectrin recruitment of ankyrin-B to costameres. Ankyrin-B then interacts with dynactin-4 and dystrophin, whereas dynactin-4 collaborates with dystrophin in coordinating costamere-aligned microtubules.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

