Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin
- PMID: 21186369
- PMCID: PMC3134999
- DOI: 10.1038/nm.2277
Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin
Abstract
The adipocyte-derived secretory factor adiponectin promotes insulin sensitivity, decreases inflammation and promotes cell survival. No unifying mechanism has yet explained how adiponectin can exert such a variety of beneficial systemic effects. Here, we show that adiponectin potently stimulates a ceramidase activity associated with its two receptors, AdipoR1 and AdipoR2, and enhances ceramide catabolism and formation of its antiapoptotic metabolite--sphingosine-1-phosphate (S1P)--independently of AMP-dependent kinase (AMPK). Using models of inducible apoptosis in pancreatic beta cells and cardiomyocytes, we show that transgenic overproduction of adiponectin decreases caspase-8-mediated death, whereas genetic ablation of adiponectin enhances apoptosis in vivo through a sphingolipid-mediated pathway. Ceramidase activity is impaired in cells lacking both adiponectin receptor isoforms, leading to elevated ceramide levels and enhanced susceptibility to palmitate-induced cell death. Combined, our observations suggest a unifying mechanism of action for the beneficial systemic effects exerted by adiponectin, with sphingolipid metabolism as its core upstream signaling component.
Figures



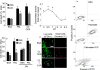

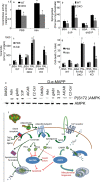
Comment in
-
Adiponectin sphings into action.Nat Med. 2011 Jan;17(1):37-8. doi: 10.1038/nm0111-37. Nat Med. 2011. PMID: 21217676 No abstract available.
-
Adiponectin receptor signaling: a new layer to the current model.Cell Metab. 2011 Feb 2;13(2):123-4. doi: 10.1016/j.cmet.2011.01.012. Cell Metab. 2011. PMID: 21284979
References
-
- Yamauchi T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. - PubMed
-
- Combs TP, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 DK086629/DK/NIDDK NIH HHS/United States
- F32 DK081279/DK/NIDDK NIH HHS/United States
- T32-HL007360/HL/NHLBI NIH HHS/United States
- R01-DK55758/DK/NIDDK NIH HHS/United States
- T32 HL007360/HL/NHLBI NIH HHS/United States
- R01 DK055758/DK/NIDDK NIH HHS/United States
- R01 CA112023/CA/NCI NIH HHS/United States
- TL1 DK081181/DK/NIDDK NIH HHS/United States
- F32 DK083866/DK/NIDDK NIH HHS/United States
- P01 DK088761/DK/NIDDK NIH HHS/United States
- F32-DK083866/DK/NIDDK NIH HHS/United States
- F32 DK085935/DK/NIDDK NIH HHS/United States
- R01 DK081456/DK/NIDDK NIH HHS/United States
- RC1-DK086629/DK/NIDDK NIH HHS/United States
- P01-DK088761/DK/NIDDK NIH HHS/United States
- F32-DK085935/DK/NIDDK NIH HHS/United States
- R01 DK056886/DK/NIDDK NIH HHS/United States
- F32-DK081279/DK/NIDDK NIH HHS/United States
- R21-DK073181/DK/NIDDK NIH HHS/United States
- R21 DK073181/DK/NIDDK NIH HHS/United States
- P01-DK49210/DK/NIDDK NIH HHS/United States
- R01-DK56886/DK/NIDDK NIH HHS/United States
- R01-CA112023/CA/NCI NIH HHS/United States
- TL1-DK081181/DK/NIDDK NIH HHS/United States
- P01 DK049210/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

