The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland
- PMID: 21187369
- PMCID: PMC3063732
- DOI: 10.1124/mol.110.069104
The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland
Abstract
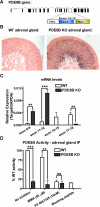
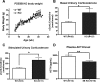
The functions of the phosphodiesterase 8B (PDE8) family of phosphodiesterases have been largely unexplored because of the unavailability of selective pharmacological inhibitors. Here, we report a novel function of PDE8B as a major regulator of adrenal steroidogenesis using a genetically ablated PDE8B mouse model as well as cell lines treated with either a new PDE8-selective inhibitor or a short hairpin RNA (shRNA) construct against PDE8B. We demonstrate that PDE8B is highly enriched in mouse adrenal fasciculata cells, and show that PDE8B knockout mice have elevated urinary corticosterone as a result of adrenal hypersensitivity toward adrenocorticotropin. Likewise, ablation of PDE8B mRNA transcripts by an shRNA construct potentiates steroidogenesis in the commonly used Y-1 adrenal cell line. We also observed that the PDE8-selective inhibitor (PF-04957325) potentiates adrenocorticotropin stimulation of steroidogenesis by increasing cAMP-dependent protein kinase activity in both primary isolated adrenocortical cells and Y-1 cells. It is noteworthy that PDE8s have their greatest control under low adrenocorticotropin-stimulated conditions, whereas other higher K(m) PDE(s) modulate steroidogenesis more effectively when cells are fully stimulated. Finally, both genetic ablation of PDE8B and long-term pharmacological inhibition of PDE8s cause increased expression of steroidogenic enzymes. We conclude that PDE8B is a major regulator of one or more pools of cAMP that promote steroidogenesis via both short- and long-term mechanisms. These findings further suggest PDE8B as a potential therapeutic target for the treatment of several different adrenal diseases.
Figures
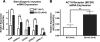
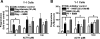
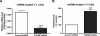
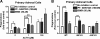
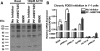

References
-
- Aguilera G, Kiss A, Liu Y, Kamitakahara A. (2007) Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress 10:153–161 - PubMed
-
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., 3rd (1997) Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 272:32656–32662 - PubMed
-
- Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR. (1995) Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem 270:11595–11602 - PubMed
-
- Chaffin CL, Dissen GA, Stouffer RL. (2000) Hormonal regulation of steroidogenic enzyme expression in granulosa cells during the peri-ovulatory interval in monkeys. Mol Hum Reprod 6:11–18 - PubMed
-
- Conti M, Beavo J. (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

