Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation
- PMID: 21187373
- PMCID: PMC3021076
- DOI: 10.1073/pnas.1012356108
Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation
Abstract
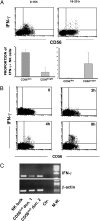
The two major functions of human natural killer (NK) cells are conventionally associated with distinct cell subsets. Thus, cytolytic activity is mostly confined to the CD56(dim)CD16(+) subset, whereas cytokine production is generally assigned to CD56(bright)CD16(+/-) cells. In this study, we reevaluated the functional capabilities of these NK subsets with regard to the production of IFN-γ at different time points after cell triggering via NKp46 and NKp30 activating receptors. Different from previous studies, cytokine production was also assessed at early intervals. We show that CD56(dim) NK cells produce IFN-γ already at 2 to 4 h, whereas no cytokine production is detected beyond 16 h. In contrast, CD56(bright) cells release IFN-γ only at late time intervals (>16 h after stimulation). The rapid IFN-γ production by CD56(dim) NK cells is in line with the presence of IFN-γ mRNA in freshly isolated cells. Rapid IFN-γ production was also induced by combinations of IL-2, IL-12, and IL-15. Our data indicate that not only cytolytic activity but also early IFN-γ production is a functional property of CD56(dim) NK cells. Thus, this subset can assure a rapid and comprehensive NK cell intervention during the early phases of innate responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15:226–233. - PubMed
-
- Moretta L, et al. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–158. - PubMed
-
- Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26:221–226. - PubMed
-
- Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16:626–633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

