Trk retrograde signaling requires persistent, Pincher-directed endosomes
- PMID: 21187387
- PMCID: PMC3021064
- DOI: 10.1073/pnas.1015981108
Trk retrograde signaling requires persistent, Pincher-directed endosomes
Abstract
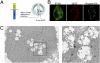
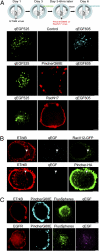
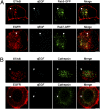
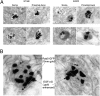
Target-derived neurotrophins use retrogradely transported Trk-signaling endosomes to promote survival and neuronal phenotype at the soma. Despite their critical role in neurotrophin signaling, the nature and molecular composition of these endosomes remain largely unknown, the result of an inability to specifically identify the retrograde signaling entity. Using EGF-bound nanoparticles and chimeric, EGF-binding TrkB receptors, we elucidate Trk-endosomal events involving their formation, processing, retrograde transport, and somal signaling in sympathetic neurons. By comparing retrograde endosomal signaling by Trk to the related but poorly neuromodulatory EGF-receptor, we find that Trk and EGF-receptor endosomes are formed and processed by distinct mechanisms. Surprisingly, Trk and EGF-receptors are both retrogradely transported to the soma in multivesicular bodies. However, only the Trk-multivesicular bodies rely on Pincher-dependent macroendocytosis and processing. Retrograde signaling through Pincher-generated Trk-multivesicular bodies is distinctively refractory to signal termination by lysosomal processing, resulting in sustained somal signaling and neuronal gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. - PubMed
-
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. - PubMed
-
- Halegoua S, Armstrong RC, Kremer NE. Dissecting the mode of action of a neuronal growth factor. Curr Top Microbiol Immunol. 1991;165:119–170. - PubMed
-
- Beattie EC, et al. A signaling endosome hypothesis to explain NGF actions: Potential implications for neurodegeneration. Cold Spring Harb Symp Quant Biol. 1996;61:389–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

