Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1
- PMID: 21187448
- PMCID: PMC3888773
- DOI: 10.4049/jimmunol.1003456
Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1
Abstract
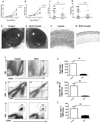
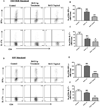
Stromal keratitis (SK) is a chronic immunopathological lesion of the eye caused by HSV-1 infection and a common cause of blindness in humans. The inflammatory lesions are primarily perpetuated by neutrophils with the active participation of CD4(+) T cells. Therefore, targeting these immune cell types represents a potentially valuable form of therapy to reduce the severity of disease. Resolvin E1 (RvE1), an endogenous lipid mediator, was shown to promote resolution in several inflammatory disease models. In the current report, we determined whether RvE1 administration begun at different times after ocular infection of mice with HSV could influence the severity of SK lesions. Treatment with RvE1 significantly reduced the extent of angiogenesis and SK lesions that occurred. RvE1-treated mice had fewer numbers of inflammatory cells that included Th1 and Th17 cells as well as neutrophils in the cornea. The mechanisms by which RvE1 acts appear to be multiple. These included reducing the influx of neutrophils and pathogenic CD4(+) T cells, increasing production of the anti-inflammatory cytokine IL-10, and inhibitory effects on the production of proinflammatory mediators and molecules, such as IL-6, IFN-γ, IL-17, KC, VEGF-A, MMP-2, and MMP-9, that are involved in corneal neovascularization and SK pathogenesis. These findings are, to our knowledge, the first to show that RvE1 treatment could represent a novel approach to control lesion severity in a virally induced immunopathological disease.
Figures






References
-
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. - PubMed
-
- Deshpande S, Banerjee K, Biswas PS, Rouse BT. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev. Mol. Med. 2004;6:1–14. - PubMed
-
- Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: An evidence-based review. Surv. Ophthalmol. 2009;54:226–234. - PubMed
-
- McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

