Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling
- PMID: 21187903
- PMCID: PMC3002989
- DOI: 10.1371/journal.ppat.1001230
Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling
Abstract
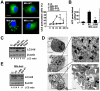
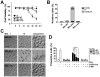
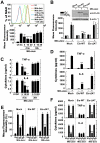
The "enhanced intracellular survival" (eis) gene of Mycobacterium tuberculosis (Mtb) is involved in the intracellular survival of M. smegmatis. However, its exact effects on host cell function remain elusive. We herein report that Mtb Eis plays essential roles in modulating macrophage autophagy, inflammatory responses, and cell death via a reactive oxygen species (ROS)-dependent pathway. Macrophages infected with an Mtb eis-deletion mutant H37Rv (Mtb-Δeis) displayed markedly increased accumulation of massive autophagic vacuoles and formation of autophagosomes in vitro and in vivo. Infection of macrophages with Mtb-Δeis increased the production of tumor necrosis factor-α and interleukin-6 over the levels produced by infection with wild-type or complemented strains. Elevated ROS generation in macrophages infected with Mtb-Δeis (for which NADPH oxidase and mitochondria were largely responsible) rendered the cells highly sensitive to autophagy activation and cytokine production. Despite considerable activation of autophagy and proinflammatory responses, macrophages infected with Mtb-Δeis underwent caspase-independent cell death. This cell death was significantly inhibited by blockade of autophagy and c-Jun N-terminal kinase-ROS signaling, suggesting that excessive autophagy and oxidative stress are detrimental to cell survival. Finally, artificial over-expression of Eis or pretreatment with recombinant Eis abrogated production of both ROS and proinflammatory cytokines, which depends on the N-acetyltransferase domain of the Eis protein. Collectively, these data indicate that Mtb Eis suppresses host innate immune defenses by modulating autophagy, inflammation, and cell death in a redox-dependent manner.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3:399–407. - PubMed
-
- Houben EN, Nguyen L, Pieters J. Interaction of pathogenic mycobacteria with the host immune system. Curr Opin Microbiol. 2006;9:76–85. - PubMed
-
- Deretic V, Fratti RA. Mycobacterium tuberculosis phagosome. Mol Microbiol. 1999;31:1603–1609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

