KIFC1-like motor protein associates with the cephalopod manchette and participates in sperm nuclear morphogenesis in Octopus tankahkeei
- PMID: 21187923
- PMCID: PMC3004946
- DOI: 10.1371/journal.pone.0015616
KIFC1-like motor protein associates with the cephalopod manchette and participates in sperm nuclear morphogenesis in Octopus tankahkeei
Abstract
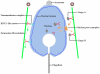
Background: Nuclear morphogenesis is one of the most fundamental cellular transformations taking place during spermatogenesis. In rodents, a microtubule-based perinuclear structure, the manchette, and a C-terminal kinesin motor KIFC1 are believed to play crucial roles in this process. Spermatogenesis in Octopus tankahkeei is a good model system to explore whether evolution has created a cephalopod prototype of mammalian manchette-based and KIFC1-dependent sperm nuclear shaping machinery.
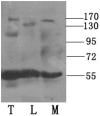
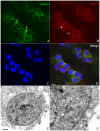
Methodology/principal findings: We detected the presence of a KIFC1-like protein in the testis, muscle, and liver of O. tankahkeei by Western Blot. Then we tracked its dynamic localization in spermatic cells at various stages using Immunofluorescence and Immunogold Electron Microscopy. The KIFC1-like protein was not expressed at early stages of spermatogenesis when no significant morphological changes occur, began to be present in early spermatid, localized around and in the nucleus of intermediate and late spermatids where the nucleus was dramatically elongated and compressed, and concentrated at one end of final spermatid. Furthermore, distribution of the motor protein during nuclear elongation and condensation overlapped with that of the cephalopod counterpart of manchette at a significant level.
Conclusions/significance: The results support the assumption that the protein is actively involved in sperm nuclear morphogenesis in O. tankahkeei possibly through bridging the manchette-like perinuclear microtubules to the nucleus and assisting in the nucleocytoplasmic trafficking of specific cargoes. This study represents the first description of the role of a motor protein in sperm nuclear shaping in cephalopod.
Conflict of interest statement
Figures





References
-
- Hess RA, Renato FL. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. - PubMed
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010a;73:241–278. - PubMed
-
- Gimenez-Bonafé P, Ribes E, Zamora MJ, Kasinsky HE, Chiva M. Evolution of octopod sperm I: comparison of nuclear morphogenesis in Eledone and Octopus. Mol Reprod Dev. 2002;62:357–362. - PubMed
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech. 2010b;73:279–319. - PubMed
-
- Dadoune JP, Siffroi JP, Alfonsi MF. Transcription in haploid male germ cells. Int Rev Cytol. 2004;237:1–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

