Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging
- PMID: 21187930
- PMCID: PMC3004955
- DOI: 10.1371/journal.pone.0015710
Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging
Erratum in
- PLoS One. 2011;6(9). doi: 10.1371/annotation/d9496d01-8c5d-4d24-8287-94449ada5064. Gunther, Matthias [added] doi: 10.1371/annotation/d9496d01-8c5d-4d24-8287-94449ada5064
Abstract
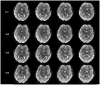
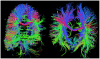
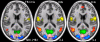
Echo planar imaging (EPI) is an MRI technique of particular value to neuroscience, with its use for virtually all functional MRI (fMRI) and diffusion imaging of fiber connections in the human brain. EPI generates a single 2D image in a fraction of a second; however, it requires 2-3 seconds to acquire multi-slice whole brain coverage for fMRI and even longer for diffusion imaging. Here we report on a large reduction in EPI whole brain scan time at 3 and 7 Tesla, without significantly sacrificing spatial resolution, and while gaining functional sensitivity. The multiplexed-EPI (M-EPI) pulse sequence combines two forms of multiplexing: temporal multiplexing (m) utilizing simultaneous echo refocused (SIR) EPI and spatial multiplexing (n) with multibanded RF pulses (MB) to achieve m×n images in an EPI echo train instead of the normal single image. This resulted in an unprecedented reduction in EPI scan time for whole brain fMRI performed at 3 Tesla, permitting TRs of 400 ms and 800 ms compared to a more conventional 2.5 sec TR, and 2-4 times reductions in scan time for HARDI imaging of neuronal fibertracks. The simultaneous SE refocusing of SIR imaging at 7 Tesla advantageously reduced SAR by using fewer RF refocusing pulses and by shifting fat signal out of the image plane so that fat suppression pulses were not required. In preliminary studies of resting state functional networks identified through independent component analysis, the 6-fold higher sampling rate increased the peak functional sensitivity by 60%. The novel M-EPI pulse sequence resulted in a significantly increased temporal resolution for whole brain fMRI, and as such, this new methodology can be used for studying non-stationarity in networks and generally for expanding and enriching the functional information.
Conflict of interest statement
Figures
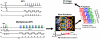


References
-
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. - PubMed
-
- Mori S, Barker PB. Diffusion magnetic resonance imaging: its principle and applications. Anat Rec. 1999;257:102–109. - PubMed
-
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. - PubMed
Publication types
MeSH terms
Grants and funding
- 1R44NS073417/NS/NINDS NIH HHS/United States
- P41 RR08079/RR/NCRR NIH HHS/United States
- P41 RR008079/RR/NCRR NIH HHS/United States
- 1U54MH091657-01/MH/NIMH NIH HHS/United States
- R44 NS073417/NS/NINDS NIH HHS/United States
- 1R44NS063537/NS/NINDS NIH HHS/United States
- R44 NS063537/NS/NINDS NIH HHS/United States
- S10 RR1395/RR/NCRR NIH HHS/United States
- P41 RR001395/RR/NCRR NIH HHS/United States
- R01 EB000331/EB/NIBIB NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- P30 NS057091/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

