The Cell Wall Teichuronic Acid Synthetase (TUAS) Is an Enzyme Complex Located in the Cytoplasmic Membrane of Micrococcus luteus
- PMID: 21188072
- PMCID: PMC3005890
- DOI: 10.1155/2010/395758
The Cell Wall Teichuronic Acid Synthetase (TUAS) Is an Enzyme Complex Located in the Cytoplasmic Membrane of Micrococcus luteus
Abstract
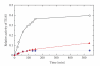
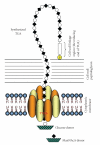
The cell wall teichuronic acid (TUA) of Micrococcus luteus is a long-chain polysaccharide composed of disaccharide repeating units [-4-β-D-ManNAcAp-(1→6)α-D-Glcp-1-](n), which is covalently anchored to the peptidoglycan on the inner cell wall and extended to the outer surface of the cell envelope. An enzyme complex responsible for the TUA chain biosynthesis was purified and characterized. The 440 kDa enzyme complex, named teichuronic acid synthetase (TUAS), is an octomer composed of two kinds of glycosyltransferases, Glucosyltransferase, and ManNAcA-transferase, which is capable of catalyzing the transfer of disaccharide glycosyl residues containing both glucose and the N-acetylmannosaminuronic acid residues. TUAS displays hydrophobic properties and is found primarily associated with the cytoplasmic membrane. The purified TUAS contains carotinoids and lipids. TUAS activity is diminished by phospholipase digestion. We propose that TUAS serves as a multitasking polysaccharide assembling station on the bacterial membrane.
Figures




References
-
- Hase S, Matsushima Y. Structural studies on a glucose-containing polysaccharideobtained from Micrococcus lysodeikticus cell walls. IV. Isolation of a disaccharide, 6-O-(strontium 2-acetamido-2-deoxy-beta-D-mannopyranosyluronate)-D-glucose. The Journal of Biochemistry. 1972;72(5):1117–1128. - PubMed
-
- Johnson SD, Lacher KP, Anderson JS. Carbon-13 nuclear magnetic resonance spectroscopic study of teichuronic acid from Micrococcus luteus cell walls. Comparison of the polysaccharide isolated from cells with that synthesized in vitro. Biochemistry. 1981;20(16):4781–4785. - PubMed
-
- Johnson GL, Hoger JH, Ratnayake JH, Anderson JS. Characterization of three intermediates in the biosynthesis of teichuronic acid of Micrococcus luteus . Archives of Biochemistry and Biophysics. 1984;235(2):679–691. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

