Role of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancer
- PMID: 21188105
- PMCID: PMC3004579
- DOI: 10.2147/cmar.s5398
Role of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancer
Abstract
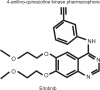
Erlotinib hydrochloride (Tarceva(®)) is a member of a class of small molecule inhibitors that targets the tyrosine kinase domain of the epidermal growth factor receptor (EGFR), with anti-tumor activity in preclinical models. Erlotinib represents a new-generation of agents known as "targeted therapies" designed to act upon cancer cells by interfering with aberrant specific activated pathways needed for tumor growth, angiogenesis and cell survival. Since its approval in November 2004 for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) after the failure of at least one prior chemotherapy regimen and with a view to improving patients' outcomes and prevent symptoms, the scientific community has evaluated the potential role of erlotinib in other scenarios such as in maintenance therapy and, in first-line setting for a selected population based on biological markers of response such as mutations of the EGFR. The convenient once-a-day pill administration and the good toxicity profile of erlotinib make it a reasonable candidate for testing in this context. This report provides a review of the role of erlotinib therapy in advanced NSCLC. It summarizes current data and perspectives of erlotinib in upfront treatment and maintenance for advanced NSCLC as well as looking at candidate biomarkers of response to these new targeted-agents.
Keywords: erlotinib; first line; maintenance; non-small-cell lung cancer; tyrosine kinase inhibitors.
Figures



References
-
- Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. - PubMed
-
- Pollack VA, Savage DM, Baker DA, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739–748. - PubMed
-
- Dancey J, Sausville EA. Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov. 2003;2:296–313. - PubMed
-
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. - PubMed
-
- EPARs for authorised medicinal products for human use, EMEA. wwwemaeuropaeu/humandocs/Humans/EPAR/tarceva/tarcevahtm Accessed March 2, 2010.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

