Hippocampal testosterone relates to reference memory performance and synaptic plasticity in male rats
- PMID: 21188275
- PMCID: PMC3006668
- DOI: 10.3389/fnbeh.2010.00187
Hippocampal testosterone relates to reference memory performance and synaptic plasticity in male rats
Abstract
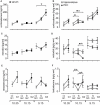
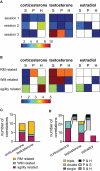
Steroids are important neuromodulators influencing cognitive performance and synaptic plasticity. While the majority of literature concerns adrenal- and gonadectomized animals, very little is known about the "natural" endogenous release of hormones during learning. Therefore, we measured blood and brain (hippocampus, prefrontal cortex) testosterone, estradiol, and corticosterone concentrations of intact male rats undergoing a spatial learning paradigm which is known to reinforce hippocampal plasticity. We found significant modulations of all investigated hormones over the training course. Corticosterone and testosterone were correlated manifold with behavior, while estradiol expressed fewer correlations. In the recall session, testosterone was tightly coupled to reference memory (RM) performance, which is crucial for reinforcement of synaptic plasticity in the dentate gyrus. Intriguingly, prefrontal cortex and hippocampal levels related differentially to RM performance. Correlations of testosterone and corticosterone switched from unspecific activity to specific cognitive functions over training. Correspondingly, exogenous application of testosterone revealed different effects on synaptic and neuronal plasticity in trained versus untrained animals. While hippocampal long-term potentiation (LTP) of the field excitatory postsynaptic potential (fEPSP) was prolonged in untrained rats, both the fEPSP- and the population spike amplitude (PSA)-LTP was impaired in trained rats. Behavioral performance was unaffected, but correlations of hippocampal field potentials with behavior were decoupled in treated rats. The data provide important evidence that besides adrenal, also gonadal steroids play a mechanistic role in linking synaptic plasticity to cognitive performance.
Keywords: LTP; dentate gyrus; endogenous; gonadal steroids; learning; stress.
Figures



Similar articles
-
Emotional and cognitive information processing: relations to behavioral performance and hippocampal long-term potentiation in vivo during a spatial water maze training in rats.Learn Mem. 2010 Oct 26;17(11):552-60. doi: 10.1101/lm.1855610. Print 2010 Nov. Learn Mem. 2010. PMID: 20978118
-
The effect of acute swim stress and training in the water maze on hippocampal synaptic activity as well as plasticity in the dentate gyrus of freely moving rats: revisiting swim-induced LTP reinforcement.Hippocampus. 2013 Dec;23(12):1291-8. doi: 10.1002/hipo.22166. Epub 2013 Aug 5. Hippocampus. 2013. PMID: 23836535
-
Impaired in vivo synaptic plasticity in dentate gyrus and spatial memory in juvenile rats induced by prenatal morphine exposure.Hippocampus. 2009 Jul;19(7):649-57. doi: 10.1002/hipo.20540. Hippocampus. 2009. PMID: 19115391
-
Neurosteroids in Adult Hippocampus of Male and Female Rodents: Biosynthesis and Actions of Sex Steroids.Front Endocrinol (Lausanne). 2018 Apr 23;9:183. doi: 10.3389/fendo.2018.00183. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29740398 Free PMC article. Review.
-
Neural sex steroids and hippocampal synaptic plasticity.Vitam Horm. 2020;114:125-143. doi: 10.1016/bs.vh.2020.06.001. Epub 2020 Jul 3. Vitam Horm. 2020. PMID: 32723541 Review.
Cited by
-
Differences in Hypothalamic Lipid Profiles of Young and Aged Male Rats With Impaired and Unimpaired Spatial Cognitive Abilities and Memory.Front Aging Neurosci. 2020 Jul 3;12:204. doi: 10.3389/fnagi.2020.00204. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32719597 Free PMC article.
-
Gestational and lactational exposition to di-n-butyl phthalate increases neurobehavioral perturbations in rats: A three generational comparative study.Toxicol Rep. 2020 Mar 19;7:480-491. doi: 10.1016/j.toxrep.2020.03.006. eCollection 2020. Toxicol Rep. 2020. PMID: 32292708 Free PMC article.
-
On the effects of testosterone on brain behavioral functions.Front Neurosci. 2015 Feb 17;9:12. doi: 10.3389/fnins.2015.00012. eCollection 2015. Front Neurosci. 2015. PMID: 25741229 Free PMC article. Review.
-
Reversal of prenatal morphine exposure-induced memory deficit in male but not female rats.J Mol Neurosci. 2013 May;50(1):58-69. doi: 10.1007/s12031-012-9860-z. Epub 2012 Aug 3. J Mol Neurosci. 2013. PMID: 22864979
-
Nk3R blockade has sex-divergent effects on memory in mice.Biol Sex Differ. 2022 Jun 11;13(1):28. doi: 10.1186/s13293-022-00437-z. Biol Sex Differ. 2022. PMID: 35690790 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

