Molecular enigma of multicolor bioluminescence of firefly luciferase
- PMID: 21188462
- PMCID: PMC11114832
- DOI: 10.1007/s00018-010-0607-0
Molecular enigma of multicolor bioluminescence of firefly luciferase
Abstract
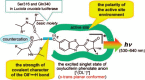
Firefly luciferase-catalyzed reaction proceeds via the initial formation of an enzyme-bound luciferyl adenylate intermediate. The chemical origin of the color modulation in firefly bioluminescence has not been understood until recently. The presence of the same luciferin molecule, in combination with various mutated forms of luciferase, can emit light at slightly different wavelengths, ranging from red to yellow to green. A historical perspective of development in understanding of color emission mechanism is presented. To explain the variation in the color of the bioluminescence, different factors have been discussed and five hypotheses proposed for firefly bioluminescence color. On the basis of recent results, light-color modulation mechanism of firefly luciferase propose that the light emitter is the excited singlet state of OL(-) [(1)(OL(-))*], and light emission from (1)(OL(-))* is modulated by the polarity of the active-site environment at the phenol/phenolate terminal of the benzothiazole fragment in oxyluciferin.
Figures



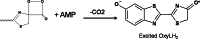






Similar articles
-
Spectroscopic studies of the light-color modulation mechanism of firefly (beetle) bioluminescence.J Am Chem Soc. 2009 Feb 18;131(6):2385-96. doi: 10.1021/ja808836b. J Am Chem Soc. 2009. PMID: 19159303
-
Glu311 and Arg337 Stabilize a Closed Active-site Conformation and Provide a Critical Catalytic Base and Countercation for Green Bioluminescence in Beetle Luciferases.Biochemistry. 2016 Aug 30;55(34):4764-76. doi: 10.1021/acs.biochem.6b00260. Epub 2016 Aug 18. Biochemistry. 2016. PMID: 27391007
-
Experimental Support for a Single Electron-Transfer Oxidation Mechanism in Firefly Bioluminescence.J Am Chem Soc. 2015 Jun 24;137(24):7592-5. doi: 10.1021/jacs.5b03820. Epub 2015 Jun 12. J Am Chem Soc. 2015. PMID: 26057379
-
The role of protein globule in firefly luciferase catalysis.Photochem Photobiol. 2024 Sep-Oct;100(5):1191-1199. doi: 10.1111/php.13909. Epub 2024 Jan 18. Photochem Photobiol. 2024. PMID: 38235806 Review.
-
Firefly luciferase: an adenylate-forming enzyme for multicatalytic functions.Cell Mol Life Sci. 2010 Feb;67(3):387-404. doi: 10.1007/s00018-009-0170-8. Epub 2009 Oct 27. Cell Mol Life Sci. 2010. PMID: 19859663 Free PMC article. Review.
Cited by
-
Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission.Int J Mol Sci. 2020 Sep 7;21(18):6538. doi: 10.3390/ijms21186538. Int J Mol Sci. 2020. PMID: 32906768 Free PMC article. Review.
-
Beetle luciferases with naturally red- and blue-shifted emission.Life Sci Alliance. 2018 Aug 16;1(4):e201800072. doi: 10.26508/lsa.201800072. eCollection 2018 Aug. Life Sci Alliance. 2018. PMID: 30456363 Free PMC article.
-
Biochemical Analysis Leads to Improved Orthogonal Bioluminescent Tools.Chembiochem. 2023 Mar 14;24(6):e202200726. doi: 10.1002/cbic.202200726. Epub 2023 Feb 10. Chembiochem. 2023. PMID: 36592373 Free PMC article.
-
Structural and dynamical insight into thermally induced functional inactivation of firefly luciferase.PLoS One. 2017 Jul 3;12(7):e0180667. doi: 10.1371/journal.pone.0180667. eCollection 2017. PLoS One. 2017. PMID: 28672033 Free PMC article.
-
Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection.Biosensors (Basel). 2022 Oct 24;12(11):918. doi: 10.3390/bios12110918. Biosensors (Basel). 2022. PMID: 36354427 Free PMC article.
References
-
- Harvey EN. Bioluminescence. New York: Academic; 1952.
-
- Haneda Y, Johnson FH, editors. Bioluminescence in progress. New Jersey: Princeton University Press; 1966.
-
- Herring PJ, editor. Bioluminescence in action. New York: Academic; 1978.
-
- Campbell AK. Chemiluminescence: principle and applications in biology and medicine. New York: VCH; 1988.
-
- Shimomura O. Bioluminescence: chemical principles and methods. Singapore: World Scientific; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

