Secretion of triacylglycerol-poor VLDL particles from McA-RH7777 cells expressing human hepatic lipase
- PMID: 21189265
- PMCID: PMC3035690
- DOI: 10.1194/jlr.M012476
Secretion of triacylglycerol-poor VLDL particles from McA-RH7777 cells expressing human hepatic lipase
Abstract
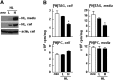
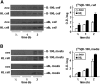
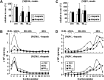
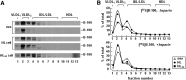
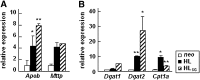
Hepatic lipase (HL) plays a role in the catabolism of apolipoprotein (apo)B-containing lipoproteins through its lipolytic and ligand-binding properties. We describe a potential intracellular role of HL in the assembly and secretion of VLDL. Transient or stable expression of HL in McA-RH7777 cells resulted in decreased (by 40%) incorporation of [(3)H]glycerol into cell-associated and secreted triacylglycerol (TAG) relative to control cells. However, incorporation of [(35)S]methionine/cysteine into cell and medium apoB-100 was not decreased by HL expression. The decreased (3)H-TAG synthesis/secretion in HL expressing cells was not attributable to decreased expression of genes involved in lipogenesis. Fractionation of medium revealed that the decreased [(3)H]TAG from HL expressing cells was mainly attributable to decreased VLDL. Expression of catalytically-inactive HL (HL(SG)) (Ser-145 at the catalytic site was substituted with Gly) in the cells also resulted in decreased secretion of VLDL-[(3)H]TAG. Examination of lumenal contents of microsomes showed a 40% decrease in [(3)H]TAG associated with lumenal lipid droplets in HL or HL(SG) expressing cells as compared with control. The microsomal membrane-associated [(3)H]TAG was decreased by 50% in HL expressing cells but not in HL(SG) expressing cells. Thus, expression of HL, irrespective of its lipolytic function, impairs formation of VLDL precursor [(3)H]TAG in the form of lumenal lipid droplets. These results suggest that HL expression in McA-RH7777 cells result in secretion of [(3)H]TAG-poor VLDL.
Figures


References
-
- Martin G. A., Busch S. J., Meredith G. D., Cardin A. D., Blankenship D. T., Mao S. J., Rechtin A. E., Woods C. W., Racke M. M., Schafer M. P., et al. 1988. Isolation and cDNA sequence of human postheparin plasma hepatic triglyceride lipase. J. Biol. Chem. 263: 10907–10914. - PubMed
-
- Sanan D. A., Fan J., Bensadoun A., Taylor J. M. 1997. Hepatic lipase is abundant on both hepatocyte and endothelial cell surfaces in the liver. J. Lipid Res. 38: 1002–1013. - PubMed
-
- Yu W., Hill J. S. 2006. Mapping the heparin-binding domain of human hepatic lipase. Biochem. Biophys. Res. Commun. 343: 659–665. - PubMed
-
- Stahnke G., Sprengel R., Augustin J., Will H. 1987. Human hepatic triglyceride lipase: cDNA cloning, amino acid sequence and expression in a cultured cell line. Differentiation. 35: 45–52. - PubMed
-
- McCoy M. G., Sun G. S., Marchadier D., Maugeais C., Glick J. M., Rader D. J. 2002. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 43: 921–929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

