Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes
- PMID: 21189319
- PMCID: PMC3067510
- DOI: 10.1128/IAI.00658-10
Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes
Abstract
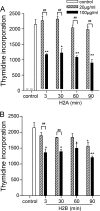
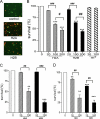
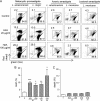
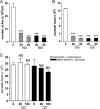
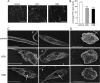
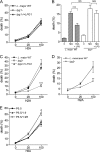
Recent studies have shown that histone proteins can act as antimicrobial peptides in host defense against extracellular bacteria, fungi, and Leishmania promastigotes. In this study, we used human recombinant histone proteins to further study their leishmaniacidal effects and the underlying mechanisms. We found that the histones H2A and H2B (but not H1(0)) could directly and efficiently kill promastigotes of Leishmania amazonensis, L. major, L. braziliensis, and L. mexicana in a treatment dose-dependent manner. Scanning electron microscopy revealed surface disruption of histone-treated promastigotes. More importantly, the preexposure of promastigotes to histone proteins markedly decreased the infectivity of promastigotes to murine macrophages (Mφs) in vitro. However, axenic and lesion-derived amastigotes of L. amazonensis and L. mexicana were relatively resistant to histone treatment, which correlated with the low levels of intracellular H2A in treated amastigotes. To understand the mechanisms underlying these differential responses, we investigated the role of promastigote surface molecules in histone-mediated killing. Compared with the corresponding controls, transgenic L. amazonensis promastigotes expressing lower levels of surface gp63 proteins were more susceptible to histone H2A, while L. major and L. mexicana promastigotes with targeted deletion of the lipophosphoglycan 2 (lpg2) gene (but not the lpg1 gene) were more resistant to histone H2A. We discuss the influence of promastigote major surface molecules in the leishmaniacidal effect of histone proteins. This study provides new information on host innate immunity to different developmental stages of Leishmania parasites.
Figures
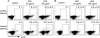
References
-
- Appelberg, R. 2007. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 15:87-92. - PubMed
-
- Bahr, V., et al. 1993. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol. Biochem. Parasitol. 58:107-121. - PubMed
-
- Brinkmann, V., et al. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532-1535. - PubMed
-
- Chan, D. I., E. J. Prenner, and H. J. Vogel. 2006. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim. Biophys. Acta 1758:1184-1202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

