Disruption of a Sirt1-dependent autophagy checkpoint in the prostate results in prostatic intraepithelial neoplasia lesion formation
- PMID: 21189328
- PMCID: PMC3033220
- DOI: 10.1158/0008-5472.CAN-10-3172
Disruption of a Sirt1-dependent autophagy checkpoint in the prostate results in prostatic intraepithelial neoplasia lesion formation
Abstract
The Sirtuin family of proteins (SIRT) encode a group of evolutionarily conserved, NAD-dependent histone deacetylases, involved in many biological pathways. SIRT1, the human homologue of the yeast Silent Information Regulator 2 (Sir2) gene, deacetylates histones, p300, p53, and the androgen receptor. Autophagy is required for the degradation of damaged organelles and long-lived proteins, as well as for the development of glands such as the breast and prostate. Herein, homozygous deletion of the Sirt1 gene in mice resulted in prostatic intraepithelial neoplasia (PIN) associated with reduced autophagy. Genome-wide gene expression analysis of Sirt1(-/-) prostates demonstrated that endogenous Sirt1 repressed androgen responsive gene expression and induced autophagy in the prostate. Sirt1 induction of autophagy occurred at the level of autophagosome maturation and completion in cultured prostate cancer cells. These studies provide novel evidence for a checkpoint function of Sirt1 in the development of PIN and further highlight a role for SIRT1 as a tumor suppressor in the prostate.
Conflict of interest statement
The authors declare no conflict of interest as they pertain to this manuscript.
Figures



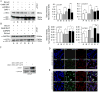

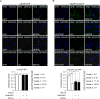

References
-
- Yang T, Fu M, Pestell R, Sauve AA. SIRT1 and endocrine signaling. Trends Endocrinol Metab. 2006;17:186–91. - PubMed
-
- Whittle JR, Powell MJ, Popov VM, Shirley LA, Wang C, Pestell RG. Sirtuins, nuclear hormone receptor acetylation and transcriptional regulation. Trends Endocrinol Metab. 2007;18:356–64. - PubMed
-
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–68. - PubMed
-
- Lim CS. SIRT1: tumor promoter or tumor suppressor? Med Hypotheses. 2006;67:341–4. - PubMed
-
- Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA075503/CA/NCI NIH HHS/United States
- R01CA120876/CA/NCI NIH HHS/United States
- R01CA086072/CA/NCI NIH HHS/United States
- R01CA137494/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- R01CA132115/CA/NCI NIH HHS/United States
- P30CA056036/CA/NCI NIH HHS/United States
- R01CA107382/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
- R01 CA075503/CA/NCI NIH HHS/United States
- R01CA070896/CA/NCI NIH HHS/United States
- R01 CA132115/CA/NCI NIH HHS/United States
- R01 CA107382/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

