Genetic control of Candida albicans biofilm development
- PMID: 21189476
- PMCID: PMC3891587
- DOI: 10.1038/nrmicro2475
Genetic control of Candida albicans biofilm development
Abstract
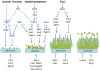
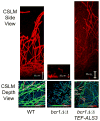
Candida species cause frequent infections owing to their ability to form biofilms - surface-associated microbial communities - primarily on implanted medical devices. Increasingly, mechanistic studies have identified the gene products that participate directly in the development of Candida albicans biofilms, as well as the regulatory circuitry and networks that control their expression and activity. These studies have uncovered new mechanisms and signals that govern C. albicans biofilm development and associated drug resistance, thus providing biological insight and therapeutic foresight.
Figures

References
-
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 36:1–53. - PubMed
-
- Pappas PG, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161–89. - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. - PubMed
-
- Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

