Improved mobilization of the CD34(+) and CD133(+) bone marrow-derived circulating progenitor cells by freshly isolated intracoronary bone marrow cell transplantation in patients with ischemic heart disease
- PMID: 21190450
- PMCID: PMC3161116
- DOI: 10.1089/scd.2010.0373
Improved mobilization of the CD34(+) and CD133(+) bone marrow-derived circulating progenitor cells by freshly isolated intracoronary bone marrow cell transplantation in patients with ischemic heart disease
Abstract
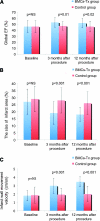
Cell therapy is a promising novel option for treatment of cardiovascular disease. Because the role of bone marrow-derived circulating progenitor cells (BM-CPCs) after cell therapy is less clear, we analyzed in this randomized, controlled study the influence of intracoronary autologous freshly isolated bone marrow cell transplantation (BMC-Tx) by using a point-of-care system on cardiac function and on the mobilization of BM-CPCs in patients with ischemic heart disease (IHD). Fifty-six patients with IHD were randomized to either receive freshly isolated BMC-Tx or a control group that did not receive cell therapy. Peripheral blood concentrations of CD34/45(+) and CD133/45(+) CPCs were measured by flow cytometry pre-, immediately post-, and at 3, 6, and 12 months postprocedure in both groups. Global ejection fraction and the size of infarct area were determined by left ventriculography. We observed in patients with IHD after intracoronary transplantation of autologous freshly isolated BMCs-Tx at 3 and 12 months follow-up a significant reduction of the size of infarct area and increase of global ejection fraction as well as infarct wall movement velocity. The mobilization of CD34/45(+) and CD133/45(+) BM-CPCs significantly increased at 3, 6, and 12 months after cell therapy when compared with baseline in patients with IHD, although no significant changes were observed between pre- and immediately postintracoronary cell therapy administration. In the control group without cell therapy, there was no significant difference of CD34/45(+) and CD133/45(+) BM-CPCs mobilization between pre- and at 3, 6, and 12 months postcoronary angiography. Intracoronary transplantation of autologous freshly isolated BMCs by using a point-of-care system in patients with IHD may enhance and prolong the mobilization of CD34/45(+) and CD133/45(+) BM-CPCs in peripheral blood and this might increase the regenerative potency in IHD.
Figures


References
-
- Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Rafii S. Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. - PubMed
-
- Peichev M. Naiyer AJ. Pereira D. Zhu Z. Lane WJ. Williams M. Oz MC. Hicklin DJ. Witte L. Moore MA. Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. - PubMed
-
- Yin AH. Miraglia S. Zanjani ED. Almeida-Porada G. Ogawa M. Leary AG. Olweus J. Kearney J. Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
