General anesthesia, sleep, and coma
- PMID: 21190458
- PMCID: PMC3162622
- DOI: 10.1056/NEJMra0808281
General anesthesia, sleep, and coma
Figures
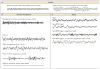
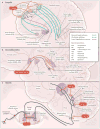
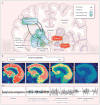
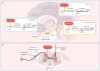
References
-
- Sentinel event alert: preventing, and managing the impact of, anesthesia awareness. Oakbrook Terrace, IL: The Joint Commission; 2004. ( http://www.jointcommission.org/sentinel_event_alert_issue_32_preventing_....) - PubMed
-
- Evers A, Crowder M. Cellular and molecular mechanisms of anesthesia. In: Barash PG, Cullen BF, Stoelting RK, Cahalan M, Stock MC, editors. Clinical anesthesia. 6. New York: Lippincott Williams & Wilkins; 2006. pp. 95–114.
-
- Gibbs FA, Gibbs LE, Lennox WG. Effects on the electroencephalogram of certain drugs which influence nervous activity. Arch Intern Med. 1937;60:154–66.
-
- Kiersey DK, Bickford RG, Faulconer A., Jr Electro-encephalographic patterns produced by thiopental sodium during surgical operations; description and classification. Br J Anaesth. 1951;23:141–52. - PubMed
-
- Watson C, Bagdoyan H, Lydic R. A neurochemical perspective on states of consciousness. In: Hudetz AG, Pearce RA, editors. Suppressing the mind: anesthetic modulation of memory and consciousness. New York: Springer/Humana Press; 2010. pp. 33–80.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
