New insights into the role of RNase L in innate immunity
- PMID: 21190483
- PMCID: PMC3021357
- DOI: 10.1089/jir.2010.0120
New insights into the role of RNase L in innate immunity
Abstract
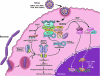
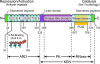
The interferon (IFN)-inducible 2'-5'-oligoadenylate synthetase (OAS)/RNase L pathway blocks infections by some types of viruses through cleavage of viral and cellular single-stranded RNA. Viruses induce type I IFNs that initiate signaling to the OAS genes. OAS proteins are pathogen recognition receptors for the viral pathogen-associated molecular pattern, double-stranded RNA. Double-stranded RNA activates OAS to produce p(x)5'A(2'p5'A)(n); x = 1-3; n > 2 (2-5A) from ATP. Upon binding 2-5A, RNase L is converted from an inactive monomer to a potently active dimeric endoribonuclease for single-stranded RNA. RNase L contains, from N- to C-terminus, a series of 9 ankyrin repeats, a linker, several protein kinase-like motifs, and a ribonuclease domain homologous to Ire1 (involved in the unfolded protein response). In the past few years, it has become increasingly apparent that RNase L and OAS contribute to innate immunity in many ways. For example, small RNA cleavage products produced by RNase L during viral infections can signal to the retinoic acid-inducible-I like receptors to amplify and perpetuate signaling to the IFN-β gene. In addition, RNase L is now implicated in protecting the central nervous system against viral-induced demyelination. A role in tumor suppression was inferred by mapping of the RNase L gene to the hereditary prostate cancer 1 (HPC1) gene, which in turn led to discovery of the xenotropic murine leukemia-related virus. A broader role in innate immunity is suggested by involvement of RNase L in cytokine induction and endosomal pathways that suppress bacterial infections. These newly described findings about RNase L could eventually provide the basis for developing broad-spectrum antimicrobial drugs.
Figures
References
-
- Allen MD. Buchberger A. Bycroft M. The PUB domain functions as a p97 binding module in human peptide N-glycanase. J Biol Chem. 2006;281(35):25502–25508. - PubMed
-
- Andersen JB. Li XL. Judge CS. Zhou A. Jha BK. Shelby S. Zhou L. Silverman RH. Hassel BA. Role of 2-5A-dependent RNase-L in senescence and longevity. Oncogene. 2007;26(21):3081–3088. - PubMed
-
- Arnold RS. Makarova N. Osunkoya AO. Suppiah S. Scott TA. Johnson NA. Bhosle SM. Liotta D. Hunter E. Marshall FF. Ly H. Molinaro RJ. Blackwell JL. Petros JA. XMRV infection in prostate cancer patients: novel serologic assay and correlation with PCR and FISH. Urology. 2010;75:755–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

