A model of the interaction between N-type and C-type inactivation in Kv1.4 channels
- PMID: 21190652
- PMCID: PMC3010008
- DOI: 10.1016/j.bpj.2010.11.011
A model of the interaction between N-type and C-type inactivation in Kv1.4 channels
Abstract
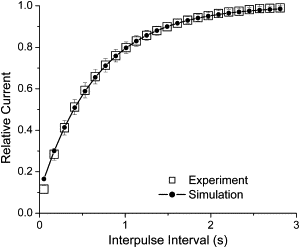
Kv1.4 channels are Shaker-related voltage-gated potassium channels with two distinct inactivation mechanisms. Fast N-type inactivation operates by a ball-and-chain mechanism. Slower C-type inactivation is not so well defined, but involves intracellular and extracellular conformational changes of the channel. We studied the interaction between inactivation mechanisms using two-electrode voltage-clamp of Kv1.4 and Kv1.4ΔN (amino acids 2-146 deleted to remove N-type inactivation) heterologously expressed in Xenopus oocytes. We manipulated C-type inactivation by introducing a lysine-tyrosine point mutation (K532Y, equivalent to Shaker T449Y) that diminishes C-type inactivation. We used experimental data to develop a comprehensive computer model of Kv1.4 channels to determine the interaction between activation and N- and C-type inactivation mechanisms needed to replicate the experimental data. C-type inactivation began at lower voltage preactivated states, whereas N-type inactivation was coupled directly to the open state. A model with distinct N- and C-type inactivated states was not able to reproduce experimental data, and direct transitions between N- and C-type inactivated states were required, i.e., there is coupling between N- and C-type inactivated states. C-type inactivation is the rate-limiting step determining recovery from inactivation, so understanding C-type inactivation, and how it is coupled to N-type inactivation, is critical in understanding how channels act to repetitive stimulation.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




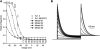





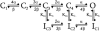




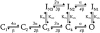

References
-
- Fergus D.J., Martens J.R., England S.K. Kv channel subunits that contribute to voltage-gated K+ current in renal vascular smooth muscle. Pflugers Arch. 2003;445:697–704. - PubMed
-
- Trimmer J.S., Rhodes K.J. Localization of voltage-gated ion channels in mammalian brain. Annu. Rev. Physiol. 2004;66:477–519. - PubMed
-
- Veh R.W., Lichtinghagen R., Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur. J. Neurosci. 1995;7:2189–2205. - PubMed
-
- Sheng M., Tsaur M.L., Jan L.Y. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. - PubMed
-
- Barry D.M., Nerbonne J.M. Myocardial potassium channels: electrophysiological and molecular diversity. Annu. Rev. Physiol. 1996;58:363–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

