Retrovirus-induced spongiform neurodegeneration is mediated by unique central nervous system viral targeting and expression of env alone
- PMID: 21191010
- PMCID: PMC3067788
- DOI: 10.1128/JVI.02210-10
Retrovirus-induced spongiform neurodegeneration is mediated by unique central nervous system viral targeting and expression of env alone
Abstract
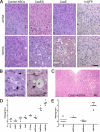
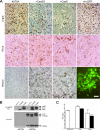
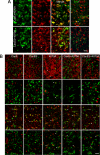
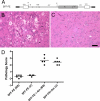
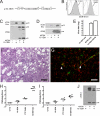
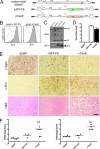
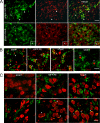
Certain murine leukemia viruses (MLVs) can induce progressive noninflammatory spongiform neurodegeneration similar to that caused by prions. The primary MLV determinants responsible have been mapped to within the env gene; however, it has remained unclear how env mediates disease, whether non-Env viral components are required, and what central nervous system (CNS) cells constitute the critical CNS targets. To address these questions, we examined the effect of transplanting engraftable C17.2 neural stem cells engineered to pseudotype, disseminate, and trans-complement neurovirulent (CasBrE, CasE, and CasES) or non-neurovirulent (Friend and SFF-FE) env sequences (SU or SU/TM) within the CNS using either the "non-neurovirulent" amphotropic helper virus, 4070A, or pgag-polgpt (a nonpackaged vector encoding Gag-Pol). These studies revealed that acute MLV-induced spongiosis results from two separable activities of Env. First, Env causes neuropathology through unique viral targeting within the CNS, which was efficiently mediated by ecotropic Envs (CasBrE and Friend), but not 4070A amphotropic Env. Second, Env induces spongiosis through a toxin activity that is MLV-receptor independent and does not require the coexpression of other viral structural proteins. CasBrE and 4070A Envs possess the toxin activity, whereas Friend Env does not. Although the identity of the critical viral target cell(s) remains unresolved, our results appear to exclude microglia and oligodendrocyte lineage cells, while implicating viral entry into susceptible neurons. Thus, MLV-induced disease parallels prionopathies in that a single protein, Env, mediates both the CNS targeting and the toxicity of the infectious agent that manifests itself as progressive vacuolar neurodegeneration.
Figures
Similar articles
-
Misfolding of CasBrE SU is reversed by interactions with 4070A Env: implications for gammaretroviral neuropathogenesis.Retrovirology. 2010 Nov 5;7:93. doi: 10.1186/1742-4690-7-93. Retrovirology. 2010. PMID: 21054857 Free PMC article.
-
Reexamination of amphotropic murine leukemia virus neurovirulence: neural stem cell-mediated microglial infection fails to induce acute neurodegeneration.Virology. 2002 Feb 15;293(2):262-72. doi: 10.1006/viro.2001.1299. Virology. 2002. PMID: 11886246
-
Late virus replication events in microglia are required for neurovirulent retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains.J Virol. 1996 Dec;70(12):8896-907. doi: 10.1128/JVI.70.12.8896-8907.1996. J Virol. 1996. PMID: 8971019 Free PMC article.
-
Neural stem cells as engraftable packaging lines can mediate gene delivery to microglia: evidence from studying retroviral env-related neurodegeneration.J Virol. 1999 Aug;73(8):6841-51. doi: 10.1128/JVI.73.8.6841-6851.1999. J Virol. 1999. PMID: 10400782 Free PMC article.
-
Differential glycosylation of the Cas-Br-E env protein is associated with retrovirus-induced spongiform neurodegeneration.J Virol. 2000 Feb;74(3):1558-65. doi: 10.1128/jvi.74.3.1558-1565.2000. J Virol. 2000. PMID: 10627570 Free PMC article. Review.
Cited by
-
Expression of HERV-Fc1, a human endogenous retrovirus, is increased in patients with active multiple sclerosis.J Virol. 2012 Apr;86(7):3713-22. doi: 10.1128/JVI.06723-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278236 Free PMC article.
-
Xpr1 is an atypical G-protein-coupled receptor that mediates xenotropic and polytropic murine retrovirus neurotoxicity.J Virol. 2012 Feb;86(3):1661-9. doi: 10.1128/JVI.06073-11. Epub 2011 Nov 16. J Virol. 2012. PMID: 22090134 Free PMC article.
-
Naturally Occurring Polymorphisms of the Mouse Gammaretrovirus Receptors CAT-1 and XPR1 Alter Virus Tropism and Pathogenicity.Adv Virol. 2011;2011:975801. doi: 10.1155/2011/975801. Epub 2011 Oct 23. Adv Virol. 2011. PMID: 22312361 Free PMC article.
-
Activation of microglia by retroviral infection correlates with transient clearance of prions from the brain but does not change incubation time.Brain Pathol. 2017 Sep;27(5):590-602. doi: 10.1111/bpa.12441. Epub 2016 Nov 21. Brain Pathol. 2017. PMID: 27558169 Free PMC article.
-
Spontaneous early-onset neurodegeneration in the brainstem and spinal cord of NSG, NOG, and NXG mice.Vet Pathol. 2023 May;60(3):374-383. doi: 10.1177/03009858231151403. Epub 2023 Feb 2. Vet Pathol. 2023. PMID: 36727841 Free PMC article.
References
-
- Andrews, J. M., and M. B. Gardner. 1974. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J. Neuropathol. Exp. Neurol. 33:285-307. - PubMed
-
- Baszler, T. V., and J. F. Zachary. 1990. Murine retroviral-induced spongiform neuronal degeneration parallels resident microglial cell infection: ultrastructural findings. Lab. Invest. 63:612-623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

