Differential effects of human immunodeficiency virus type 1 Vpu on the stability of BST-2/tetherin
- PMID: 21191020
- PMCID: PMC3067951
- DOI: 10.1128/JVI.02080-10
Differential effects of human immunodeficiency virus type 1 Vpu on the stability of BST-2/tetherin
Abstract
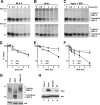
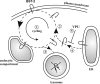
BST-2/CD317/tetherin is a host factor that inhibits the release of HIV-1 and other unrelated viruses. A current model proposes that BST-2 physically tethers virions to the surface of virus-producing cells. The HIV-1-encoded Vpu protein effectively antagonizes the activity of BST-2. How Vpu accomplishes this task remains unclear; however, it is known that Vpu has the ability to down-modulate BST-2 from the cell surface. Here we analyzed the effects of Vpu on BST-2 by performing a series of kinetic studies with HeLa, 293T, and CEMx174 cells. Our results indicate that the surface downregulation of BST-2 is not due to an accelerated internalization or reduced recycling of internalized BST-2 but instead is caused by interference with the resupply of newly synthesized BST-2 from within the cell. While our data confirm previous reports that the high-level expression of Vpu can cause the endoplasmic reticulum (ER)-associated degradation of BST-2, we found no evidence that Vpu targets endogenous BST-2 in the ER in the course of a viral infection. Instead, we found that Vpu acts in a post-ER compartment and increases the turnover of newly synthesized mature BST-2. Our observation that Vpu does not affect the recycling of BST-2 suggests that Vpu does not act directly at the cell surface but may interfere with the trafficking of newly synthesized BST-2 to the cell surface, resulting in the accelerated targeting of BST-2 to the lysosomal compartment for degradation.
Figures

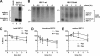


References
-
- Asaoka, K., et al. 2005. A retrovirus restriction factor TRIM5alpha is transcriptionally regulated by interferons. Biochem. Biophys. Res. Commun. 338:1950-1956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

