NHERF1 and NHERF2 are necessary for multiple but usually separate aspects of basal and acute regulation of NHE3 activity
- PMID: 21191106
- PMCID: PMC3074635
- DOI: 10.1152/ajpcell.00119.2010
NHERF1 and NHERF2 are necessary for multiple but usually separate aspects of basal and acute regulation of NHE3 activity
Abstract
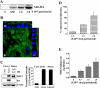
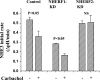
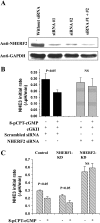
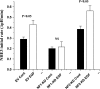
Na(+)/H(+) exchanger 3 (NHE3) is expressed in the brush border (BB) of intestinal epithelial cells and accounts for the majority of neutral NaCl absorption. It has been shown that the Na(+)/H(+) exchanger regulatory factor (NHERF) family members of multi-PDZ domain-containing scaffold proteins bind to the NHE3 COOH terminus and play necessary roles in NHE3 regulation in intestinal epithelial cells. Most studies of NHE3 regulation have been in cell models in which NHERF1 and/or NHERF2 were overexpressed. We have now developed an intestinal Na(+) absorptive cell model in Caco-2/bbe cells by expressing hemagglutinin (HA)-tagged NHE3 with an adenoviral infection system. Roles of NHERF1 and NHERF2 in NHE3 regulation were determined, including inhibition by cAMP, cGMP, and Ca(2+) and stimulation by EGF, with knockdown (KD) approaches with lentivirus (Lenti)-short hairpin RNA (shRNA) and/or adenovirus (Adeno)-small interfering RNA (siRNA). Stable infection of Caco-2/bbe cells by NHERF1 or NHERF2 Lenti-shRNA significantly and specifically reduced NHERF protein expression by >80%. NHERF1 KD reduced basal NHE3 activity, while NHERF2 KD stimulated NHE3 activity. siRNA-mediated (transient) and Lenti-shRNA-mediated (stable) gene silencing of NHERF2 (but not of NHERF1) abolished cGMP- and Ca(2+)-dependent inhibition of NHE3. KD of NHERF1 or NHERF2 alone had no effect on cAMP inhibition of NHE3, but KD of both simultaneously abolished the effect of cAMP. The stimulatory effect of EGF on NHE3 was eliminated in NHERF1-KD but occurred normally in NHERF2-KD cells. These findings show that both NHERF2 and NHERF1 are involved in setting NHE3 activity. NHERF2 is necessary for cGMP-dependent protein kinase (cGK) II- and Ca(2+)-dependent inhibition of NHE3. cAMP-dependent inhibition of NHE3 activity requires either NHERF1 or NHERF2. Stimulation of NHE3 activity by EGF is NHERF1 dependent.
Figures




Similar articles
-
Both NHERF3 and NHERF2 are necessary for multiple aspects of acute regulation of NHE3 by elevated Ca2+, cGMP, and lysophosphatidic acid.Am J Physiol Gastrointest Liver Physiol. 2018 Jan 1;314(1):G81-G90. doi: 10.1152/ajpgi.00140.2017. Epub 2017 Sep 7. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 28882822 Free PMC article.
-
NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum.Am J Physiol Cell Physiol. 2011 Jul;301(1):C126-36. doi: 10.1152/ajpcell.00311.2010. Epub 2011 Mar 23. Am J Physiol Cell Physiol. 2011. PMID: 21430287 Free PMC article.
-
D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process.Gastroenterology. 2011 Feb;140(2):560-71. doi: 10.1053/j.gastro.2010.10.042. Epub 2010 Oct 23. Gastroenterology. 2011. PMID: 20977906 Free PMC article.
-
NHERF family and NHE3 regulation.J Physiol. 2005 Aug 15;567(Pt 1):3-11. doi: 10.1113/jphysiol.2005.090399. Epub 2005 May 19. J Physiol. 2005. PMID: 15905209 Free PMC article. Review.
-
The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins.Clin Exp Pharmacol Physiol. 2008 Aug;35(8):863-71. doi: 10.1111/j.1440-1681.2008.04931.x. Epub 2008 Apr 21. Clin Exp Pharmacol Physiol. 2008. PMID: 18430067 Review.
Cited by
-
An old herbal medicine with a potentially new therapeutic application in inflammatory bowel disease.Int J Clin Exp Med. 2011;4(4):309-19. Epub 2011 Oct 29. Int J Clin Exp Med. 2011. PMID: 22140602 Free PMC article.
-
Phosphorylation and subcellular localization of Na+/H+ exchanger isoform 3 (NHE3) are associated with altered gallbladder absorptive function after formation of cholesterol gallstones.J Physiol Biochem. 2017 Feb;73(1):133-139. doi: 10.1007/s13105-016-0533-1. Epub 2016 Nov 7. J Physiol Biochem. 2017. PMID: 27822917
-
Myosin VI mediates the movement of NHE3 down the microvillus in intestinal epithelial cells.J Cell Sci. 2014 Aug 15;127(Pt 16):3535-45. doi: 10.1242/jcs.149930. Epub 2014 Jun 13. J Cell Sci. 2014. PMID: 24928903 Free PMC article.
-
SLC26A3 (DRA) is stimulated in a synergistic, intracellular Ca2+-dependent manner by cAMP and ATP in intestinal epithelial cells.Am J Physiol Cell Physiol. 2023 Jun 1;324(6):C1263-C1273. doi: 10.1152/ajpcell.00523.2022. Epub 2023 May 8. Am J Physiol Cell Physiol. 2023. PMID: 37154494 Free PMC article.
-
Phosphorylation of NHE3-S719 regulates NHE3 activity through the formation of multiple signaling complexes.Mol Biol Cell. 2017 Jul 1;28(13):1754-1767. doi: 10.1091/mbc.E16-12-0862. Epub 2017 May 11. Mol Biol Cell. 2017. PMID: 28495796 Free PMC article.
References
-
- Ahn W, Kim KH, Lee JA, Kim JY, Choi JY, Moe OW, Milgram SL, Muallem S, Lee MG. Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3− salvage mechanisms in model systems and the mouse pancreatic duct. J Biol Chem 276: 17236–17243, 2001 - PubMed
-
- Akhter S, Cavet ME, Tse CM, Donowitz M. C-terminal domains of Na+/H+ exchanger isoform 3 are involved in the basal and serum-stimulated membrane trafficking of the exchanger. Biochemistry 39: 1990–2000, 2000 - PubMed
-
- Broere N, Chen M, Cinar A, Singh AK, Hillesheim J, Riederer B, Lunnemann M, Rottinghaus I, Krabbenhoft A, Engelhardt R, Rausch B, Weinman EJ, Donowitz M, Hubbard A, Kocher O, de Jonge HR, Hogema BM, Seidler U. Defective jejunal and colonic salt absorption and altered Na+/H+ exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflügers Arch 457: 1079–1091, 2009 - PMC - PubMed
-
- Broere N, Hillesheim J, Tuo B, Jorna H, Houtsmuller AB, Shenolikar S, Weinman EJ, Donowitz M, Seidler U, de Jonge HR, Hogema BM. Cystic fibrosis transmembrane conductance regulator activation is reduced in the small intestine of Na+/H+ exchanger 3 regulatory factor 1 (NHERF-1)- but not NHERF-2-deficient mice. J Biol Chem 282: 37575–37584, 2007 - PubMed
-
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401: 286–290, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

