Noninvasive in vivo imaging to evaluate immune responses and antimicrobial therapy against Staphylococcus aureus and USA300 MRSA skin infections
- PMID: 21191403
- PMCID: PMC3059384
- DOI: 10.1038/jid.2010.417
Noninvasive in vivo imaging to evaluate immune responses and antimicrobial therapy against Staphylococcus aureus and USA300 MRSA skin infections
Abstract
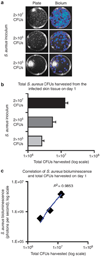
Staphylococcus aureus skin infections represent a significant public health threat because of the emergence of antibiotic-resistant strains such as methicillin-resistant S. aureus (MRSA). As greater understanding of protective immune responses and more effective antimicrobial therapies are needed, a S. aureus skin wound infection model was developed in which full-thickness scalpel cuts on the backs of mice were infected with a bioluminescent S. aureus (methicillin sensitive) or USA300 community-acquired MRSA strain and in vivo imaging was used to noninvasively monitor the bacterial burden. In addition, the infection-induced inflammatory response was quantified using in vivo fluorescence imaging of LysEGFP mice. Using this model, we found that both IL-1α and IL-1β contributed to host defense during a wound infection, whereas IL-1β was more critical during an intradermal S. aureus infection. Furthermore, treatment of a USA300 MRSA skin infection with retapamulin ointment resulted in up to 85-fold reduction in bacterial burden and a 53% decrease in infection-induced inflammation. In contrast, mupirocin ointment had minimal clinical activity against this USA300 strain, resulting in only a 2-fold reduction in bacterial burden. Taken together, this S. aureus wound infection model provides a valuable preclinical screening method to investigate cutaneous immune responses and the efficacy of topical antimicrobial therapies.
Conflict of interest statement
The authors state no conflict of interest.
Figures




References
-
- Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9–17. - PubMed
-
- Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 Suppl 5:S344–S349. - PubMed
-
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

