Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection
- PMID: 21192802
- PMCID: PMC3022836
- DOI: 10.1186/1471-2334-10-363
Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection
Abstract
Background: Oral vancomycin (125 mg qid) is recommended as treatment of severe Clostridium difficile infection (CDI). Higher doses (250 or 500 mg qid) are sometimes recommended for patients with very severe CDI, without supporting clinical evidence. We wished to determine to what extent faecal levels of vancomycin vary according to diarrhoea severity and dosage, and whether it is rational to administer high-dose vancomycin to selected patients.
Methods: We recruited hospitalized adults suspected to have CDI for whom oral vancomycin (125, 250 or 500 mg qid) had been initiated. Faeces were collected up to 3 times/day and levels were measured with the AxSYM fluorescence polarization immunoassay.
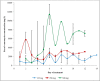
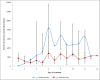
Results: Fifteen patients (9 with confirmed CDI) were treated with oral vancomycin. Patients with ≥ 4 stools daily presented lower faecal vancomycin levels than those with a lower frequency. Higher doses of oral vancomycin (250 mg or 500 mg qid) led to consistently higher faecal levels (> 2000 mg/L), which were 3 orders of magnitude higher than the MIC90 of vancomycin against C. difficile. One patient receiving 125 mg qid had levels below 50 mg/L during the first day of treatment.
Conclusions: Faecal levels of vancomycin are proportional to the dosage administered and, even in patients with increased stool frequency, much higher than the MIC90. Patients given the standard 125 mg qid dosage might have low faecal levels during the first day of treatment. A loading dose of 250 mg or 500 mg qid during the first 24-48 hours followed by the standard dosage should be evaluated in larger studies, since it might be less disruptive to the colonic flora and save unnecessary costs.
Figures
References
-
- Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M. et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

