Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation
- PMID: 21192804
- PMCID: PMC3022573
- DOI: 10.1186/1471-213X-10-122
Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation
Abstract
Background: Mammary glands harbor a profound burden of apoptotic cells (ACs) during post-lactational involution, but little is known regarding mechanisms by which ACs are cleared from the mammary gland, or consequences if this process is interrupted. We investigated AC clearance, also termed efferocytosis, during post-lactational remodeling, using mice deficient for MerTK, Axl, and Tyro3, three related receptor tyrosine kinases (RTKs) regulating macrophage-mediated efferocytosis in monocytes. MerTK expression, apoptosis and the accumulation of apoptotic debris were examined in histological sections of MerTK-deficient, Axl/Tyro3-deficient, and wild-type mammary glands harvested at specific time points during lactation and synchronized involution. The ability of primary mammary epithelial cells (MECs) to engulf ACs was assessed in culture. Transplant of MerTK-deficient mammary epithelium into cleared WT mammary fat pads was used to assess the contribution of WT mammary macrophages to post-lactational efferocytosis.
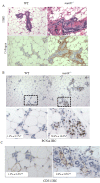
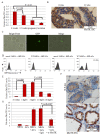
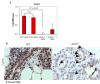
Results: ACs induced MerTK expression in MECs, resulting in elevated MerTK levels at the earliest stages of involution. Loss of MerTK resulted in AC accumulation in post-lactational MerTK-deficient mammary glands, but not in Axl and Tyro3-deficient mammary glands. Increased vascularization, fibrosis, and epithelial hyperproliferation were observed in MerTK-deficient mammary glands through at least 60 days post-weaning, due to failed efferocytosis after lactation, but did not manifest in nulliparous mice. WT host-derived macrophages failed to rescue efferocytosis in transplanted MerTK-deficient mammary epithelium.
Conclusion: Efferocytosis by MECs through MerTK is crucial for mammary gland homeostasis and function during the post-lactational period. Efferocytosis by MECs thus limits pathologic consequences associated with the apoptotic load following lactation.
Figures




References
-
- Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6(2):R75–91. doi: 10.1186/bcr753. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

